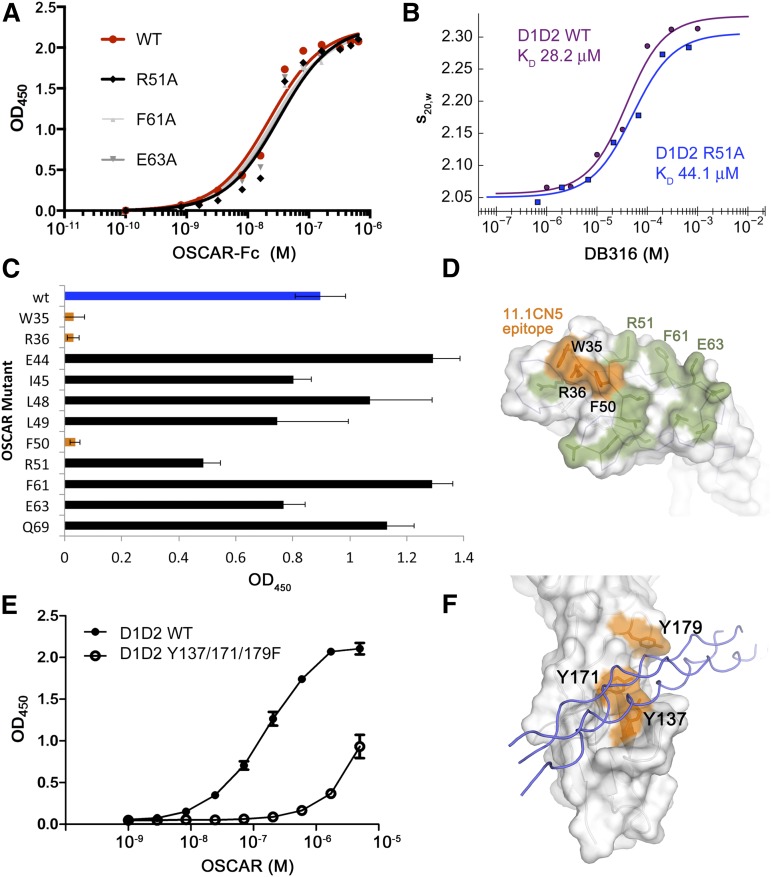
Figure 7.
Binding and mutagenesis assays reveal D2 as the primary THP-binding site. (A) Solid-phase plate-binding assay to solubilized human placental collagen I showing WT OSCAR-Fc and 3 of the alanine point mutants in the D1 THP site. All other D1 mutants showed nearly identical binding curves. (B) Solution-phase binding assay between monomeric WT D1D2 or R51A-D1D2 and DB316 analyzed by sedimentation velocity AUC. Weight-averaged values of s20,w plotted vs DB316 concentration were fitted to a single-site binding equation. (C) Binding of WT and mutant OSCAR-Fc proteins to immobilized 11.1CN5, a nonblocking anti-OSCAR mAb. (D) Surface diagram of OSCAR D1, showing the epitope for 11.1CN5 that is composed of residues Trp-35, Arg-36, and Phe-50, which overlay the D1 region of LAIR-1 implicated in collagen binding. All other tested alanine mutations are shown in green; none caused a significant loss in affinity for 11.1CN5. (E) Solid-phase binding assay with immobilized THP DB80, comparing the affinity of monomeric WT D1D2 and a triple Tyr-to-Phe mutant that targets the D2 THP-binding site observed in the OSCAR complex. The triple mutant showed 50-fold decreased affinity for DB80, confirming that OSCAR D2 contains the primary binding site for the collagen consensus sequence. (F) Surface diagram of OSCAR D2, showing the location of the residues in the triple mutant (orange) in relation to the DB316 THP (blue coils) observed in the crystal structure.