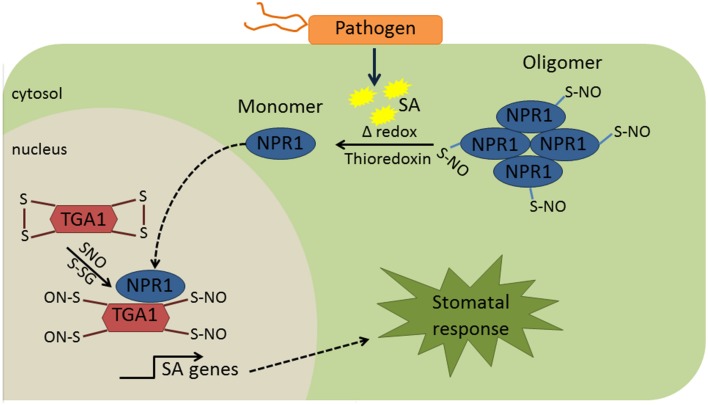
Figure 2.
Redox regulation of NPR1 and TGA1. Under normal conditions, NPR1 is retained in the cytosol as an oligomer. S-nitrosylation of NPR1 is known to promote NPR1 oligomerization. In the presence of pathogen, production of SA promotes cellular redox changes, which will contribute to reduction of the NPR1 oligomer to monomeric form. Monomeric form of NPR1 moves to the nucleus and binds to TGA1 that was nitrosylated due to cellular redox changes mediated by SA. The complex NPR1-TGA1 turns on the transcription of PR genes. Although this mechanism was not directly elucidated in the guard cells, it is likely to be the case since NPR1 was primarily in the cytosol and nucleus of guard cells (Kinkema et al., 2000). SA, salicylic acid; NPR1, nonexpresser of PR gene 1; TGA1, teosine glume architecture 1; SNO, S-nitrosylation; S-GS, S-glutathionylation.