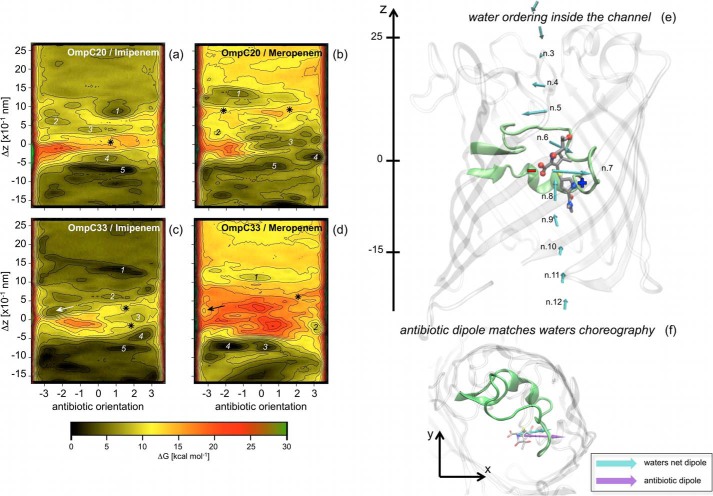
FIGURE 4.
Free energy surface of carbapenem translocation, imipenem (a and c) and meropenem (b and d) in the OmpC20 (a and b) and OmpC33 (c and d) porin are shown. The antibiotic orientation is defined as the difference of the z-coordinate between the lactam carbonyl carbon and the sulfur bonded carbon of the antibiotic two-ring core. The difference of the z-coordinate between the center of mass of the antibiotic two-ring core and that of the monomer of the porin (Δz) represents the antibiotic depth inside the porin lumen (Δz = 0 corresponds to the constriction region). Each iso contour corresponds to a free energy increase of 2 kcal mol−1. Free energy values were rescaled to have the absolute minimum equal to zero. Different labels are used to indicate specific regions analyzed and discussed in the text. Water ordering inside (e) the first monomer of OmpC20 is shown together with the net electric dipole of the water molecules calculated at different depth inside the lumen, according to a recent theoretical investigation (20). The loop L3 is highlighted to provide a reference. Meropenem is shown at the constriction region, i.e. the representative conformer for the free energy minimum 3 in Fig. 4b. Net electric charges are indicated for clarity. In f, the system is rotated to show the xy projection. The electric dipole of the antibiotic (purple) is shown together with the net dipole of water molecules (cyan) in the absence of antibiotic at the same level inside the channel.