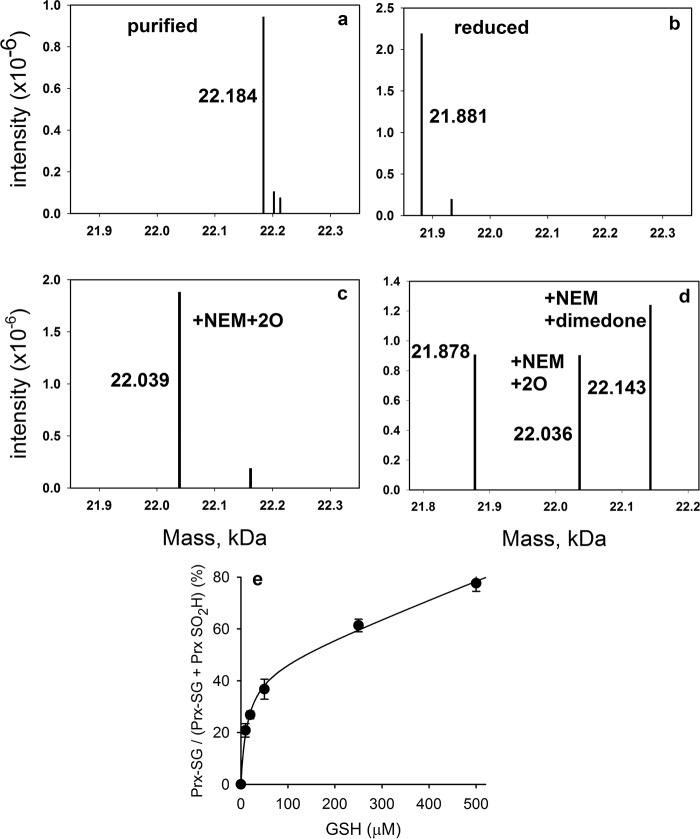
FIGURE 2.
Reactions of the C172S mutant of Prx2 with H2O2 and GSH. Deconvoluted mass spectra of the C172S mutant as isolated from E. coli under non-reducing conditions (a), following reduction with DTT and showing loss of 303 Da (b), following treatment of reduced C172S (5 μm) with a 2-fold excess of H2O2 (c), and showing trapping of the sulfenic acid with dimedone (50 mm) present during addition of equimolar H2O2 (d) are shown. e, concentration-dependent glutathionylation of CysP of C172S mutant of Prx2 and corresponding inhibition of hyperoxidation. Purified recombinant C172S Prx2 (5 μm) was reduced, mixed with varying concentrations of GSH, and subjected to reaction with 10 μm H2O2. Data represent means ± S.D. (error bars) from four individual experiments. Kinetic analysis of the data was performed using Berkeley Madonna (as in Ref. 20) and rate constants for Reactions 1 and 2 in Table 2. kGSH was varied to give the best fit (plotted), which was obtained with a value of 500 m−1 s−1.