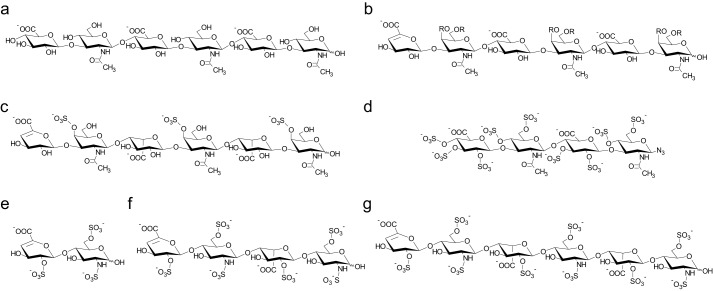
FIGURE 1.
GAGs used for NMR chemical shift perturbation experiments. The structures of HA hexasaccharide (a), CS hexasaccharide (b), DS hexasaccharide (c), and psHA tetrasaccharide (d) are shown. For heparin, different chain lengths were tested: di- (e), tetra- (f), and hexasaccharide (g). CS, DS, and heparin molecules carried a 4,5-unsaturated uronic acid ring at the non-reducing end as a result of preparation by lyase digestion. CS had a non-uniform sulfation pattern with the residue R at the O4 or O6 position being either H or SO3−. psHA was synthesized with an azide group at the reducing end.