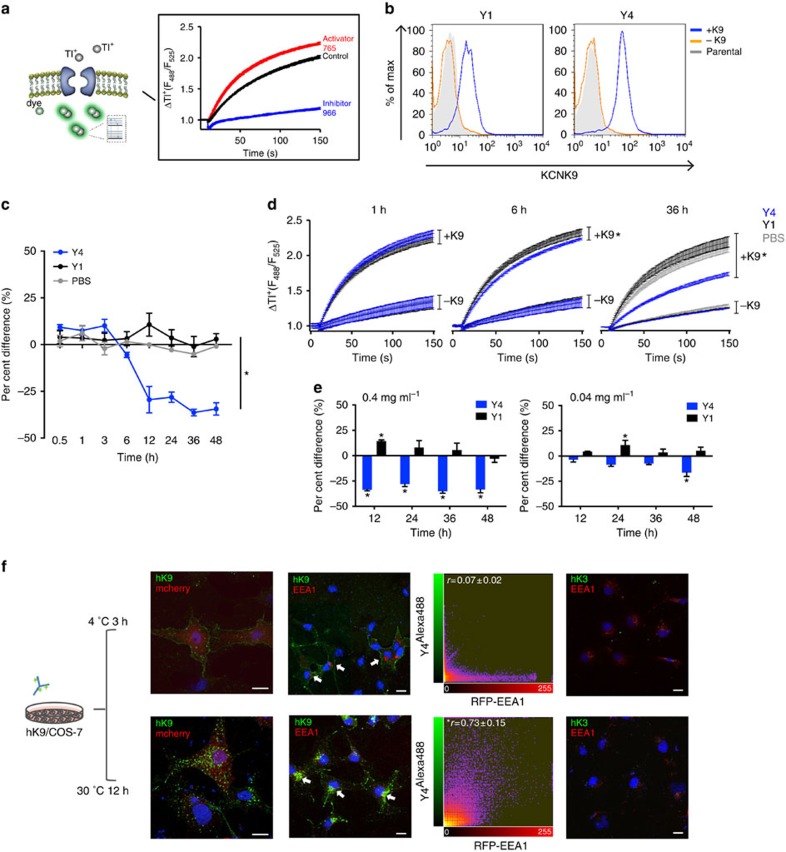
Figure 3. Y4 inhibits hKCNK9 by inducing internalization of channel from cell surface.
(a) Schematic representation of Tl+-based fluorescence assay principle (FluxOR Potassium Ion Channel Assay, Invitrogen) and readout. Compound 765 and 966 were chemical modulators used as control compounds. (b) Flow cytometry analysis of tetracycline-inducible hKCNK9 stable cell line stained with Y1 and Y4 in the presence of tetracycline induction, designated as +K9 or in the absence of tetracycline induction, designated as −K9. Parental HEK293 cells were used as negative control. (c) Time-dependent inhibition of Tl+ conductance by Y4. Tetracycline-induced cells were treated with Y4, Y1 or PBS before Tl+ assay and compared with cells without treatment. Difference in Tl+ conductance at time point 80 was used for comparison. Mean±s.d., n=24 (the experiment was replicated four times), *P<0.0001, one-way analysis of variance (ANOVA). (d) Representative Tl+ traces from tetracycline-induced KCNK9-expressing cells (+K9) and non-induced cells (−K9) plotted as fluorescence change over time. Mean±s.d., n=24 (the experiment was replicated four times), *P<0.01, two-tailed Student's t-test. (e) Concentration-dependent inhibition of Tl+ conductance by Y4. Mean±s.d., n=6 (the experiment was replicated twice), *P<0.01, two-tailed Student's t-test. (f) Internalization of hKCNK9 from cell surface induced by Y4. COS-7 cells transiently expressing hKCNK9 and mcherry or red fluorescent protein (RFP)-tagged EEA1 were incubated with Alexa488-conjugated Y4. Cells transiently expressing hKCNK3 and RFP-tagged EEA1 were control. Nuclei were counterstained with 4,6-diamidino-2-phenylindole (DAPI). Co-localization (arrows) between Alexa488-conjugated Y4 and RFP-tagged EEA1 was analysed using Imaris software and representative scatter plots were shown. Statistical analysis was based on Pearson's correlation coefficient calculated from 20 fields, mean±s.d. *r>0.5. Scale bar, 20 μm.