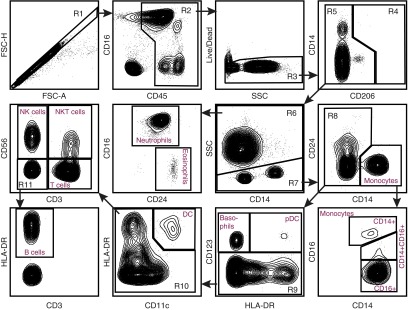
Figure 2.
Flow cytometry panel of human whole blood. Flow cytometry was performed on whole blood cells. After obtaining the sample, the red cells underwent lysis. The remaining cells underwent antibody staining per protocol. The cells were separated by FSC-A and FSC-H to obtain singlets (R1). From R1, the cells were analyzed for CD45 expression. CD45+ cells (R2) then underwent Live/Dead staining, and live cells (R3) were selected for analysis of CD206 staining. Because lung macrophages do not exist in the whole blood, there were no CD206+ cells. CD206− cells (R5) were separated into SSC high (R6) granulocytes and SSC low (R7) nongranulocytes. From the granulocytes (R6), neutrophils (CD16+CD24−) and eosinophils (CD16−CD24+) were defined. The nongranulocytes (R7) were separated into CD14+ monocytes and CD14− nonmonocytes (R8). The monocytes were then delineated on the basis of CD16 expression. Nonmonocytes (R8) were separated based on CD123 expression. CD123+ basophils and pDC were defined based on HLA-DR expression. CD123− cells (R9) were separated into CD11c+HLA-DR+ DC and CD11c− (R10) populations. This population reflected lymphocytes and B cells. Lymphocyte subsets were separated by CD3 and CD56 staining into NK cells (CD56+CD3−), natural killer T (NKT) cells (CD56+CD3+), T cells (CD56−CD3+), and double-negative cells (R11). From this group (R11), HLA-DR+ cells were defined as B Cells. Flow panel is representative of n = 3 samples of whole blood.