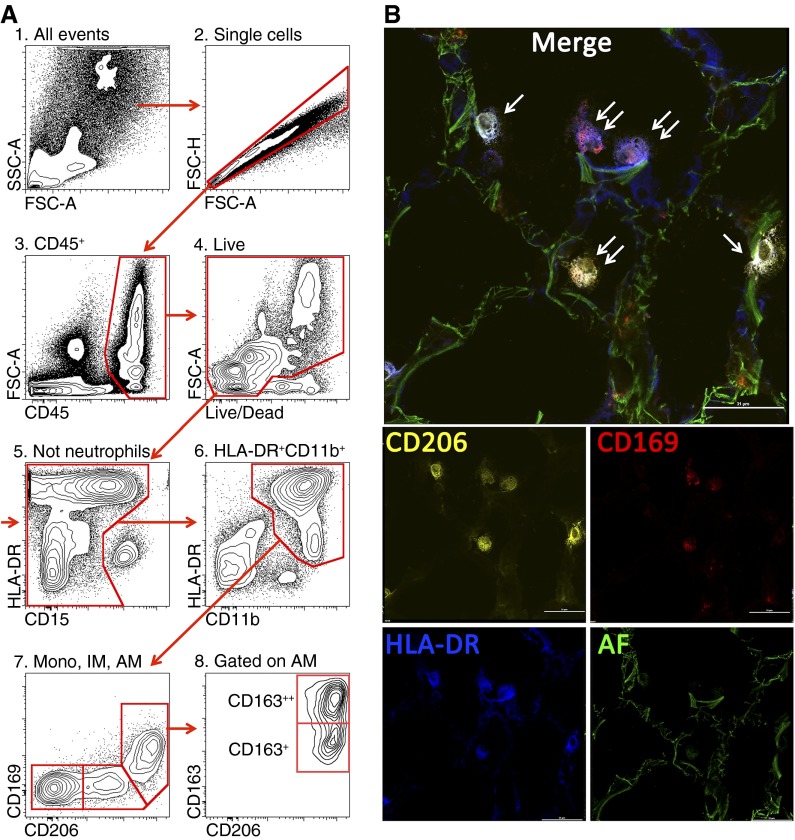
Figure 1.
(A) Gating strategy used to identify lung monocytes and macrophages. After excluding doublets (2), cells of hematopoietic origin were identified as CD45+ (3), followed by exclusion of the dead cells (precautions were taken not to gate out highly autofluorescent alveolar macrophages) (4). Neutrophils were identified as CD11b++CD15+CD16+HLA-DR− cells and excluded from analysis (5). We then gated on CD11b++HLA-DR+ cells (6). This allows separation from natural killer (NK) cells (CD11b+HLA-DR−CD56+) and highly autofluorescent eosinophils (CD11b+/−HLA-DR−Siglec 8+). Finally, using CD206 and CD169, cells were separated into three subpopulations: alveolar macrophages (CD11b+HLA-DR++CD206++CD169+FSChighSSChigh, AM), interstitial macrophages (CD11b+HLA-DR++CD206+CD169−, IM), and monocytes (CD11b+HLA-DR+CD206−CD169−, mono) (7). CD163 identifies two subpopulations of alveolar macrophages (8). (B) Immunofluorescent microscopy on a normal human lung. Orange is CD206 PE, red is CD169 AF647, blue is HLA-DR BV421, and green is autofluorescence in fluorescein isothiocyanate channel. Single arrows indicate HLA-DR+CD206+CD169− cells (interstitial macrophages), and double arrows indicate HLA-DR+CD206+CD169+ cells (alveolar macrophages). Scale bar is 31 μm.