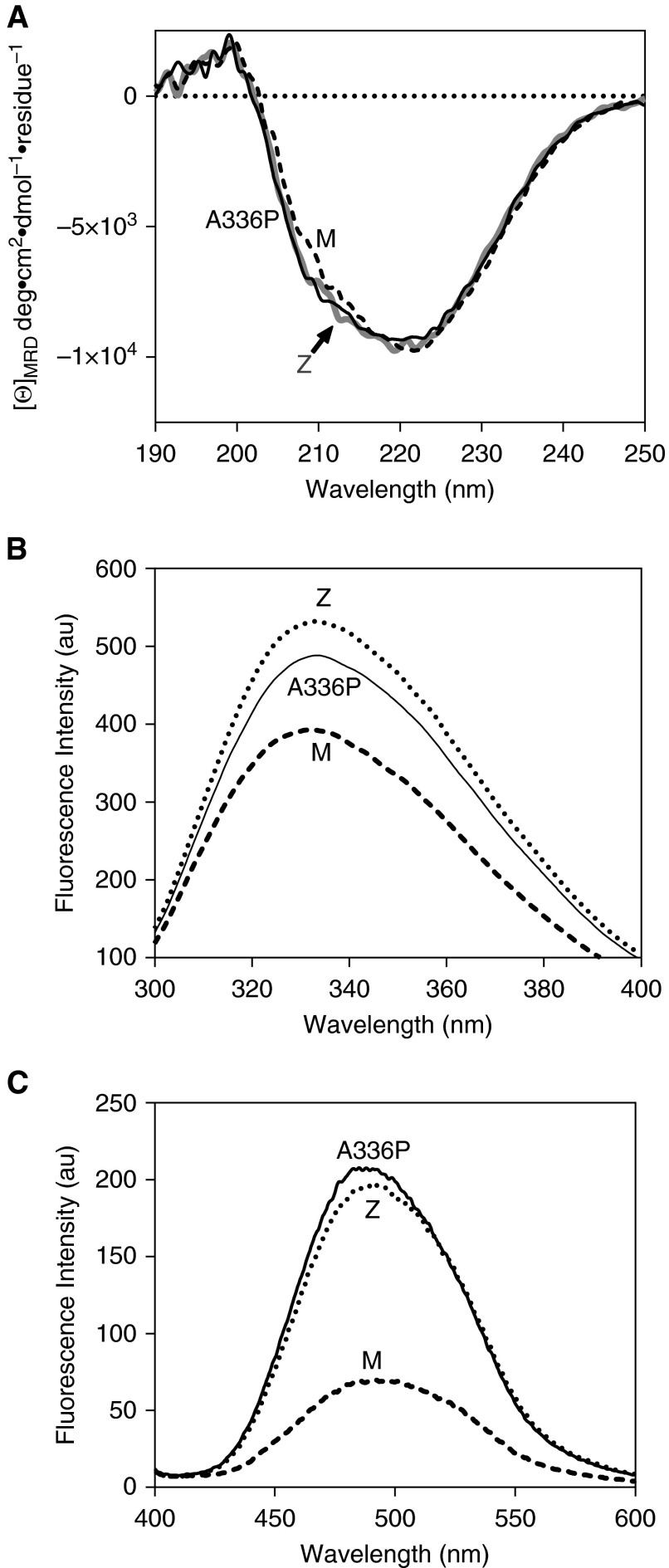
Figure 2.
Structural features of Ala336Pro. (A) Assessment of overall secondary structure by far-ultraviolet circular dichroism (CD). CD spectra of plasma-derived alpha-1 antitrypsin, at 0.5 mg/ml in 10 mM Na2HPO4/NaH2PO4 (pH 7.4) recorded between 250 and 190 nm show similar profiles for the three variants. (B) Assessment of breach opening (30) by intrinsic tryptophan fluorescence. Spectra were recorded of 0.5 μM alpha-1 antitrypsin in PBS with 5% vol/vol glycerol between 300 and 400 nm with excitation at 295 nm. Ala336Pro shows increased fluorescence intensity and red shift in wavelength compared with ATM but to a lesser extent than ATZ alpha-1 antitrypsin. (C) Alpha-1 antitrypsin (2 μM) in PBS with 5% vol/vol glycerol was incubated with 10 μM 4,4′-dianilino-1,1′-binaphthyl-5,5′-disulfonic acid for 10 minutes, and the spectra were recorded between 400 and 600 nm with excitation at 370 nm. The increase in fluorescence was comparable to ATZ, suggesting increased population of the polymerization intermediate (34). [Θ]MRD, mean residue ellipticity.