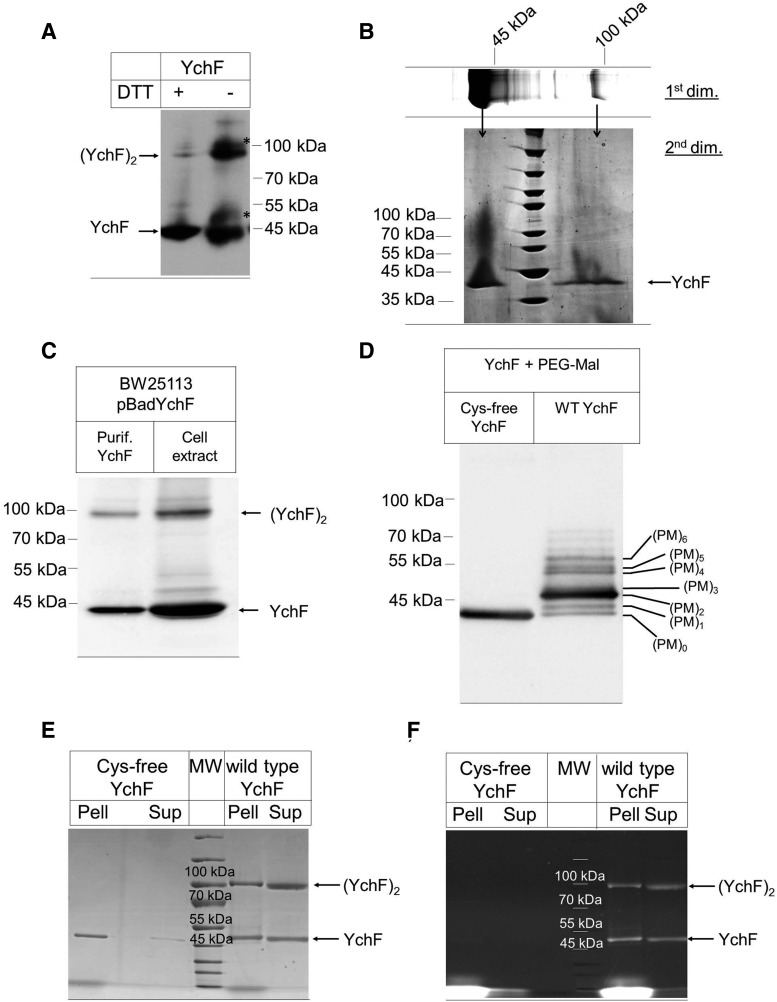
FIG. 2.
YchF forms redox- and cysteine-dependent physiological dimers. (A) Purified YchF was denatured at 37°C for 15 min at either reducing (+25 mM DTT) or nonreducing conditions (−DTT) and after sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and Western transfer probed with α-YchF antibodies. Two weaker bands were also recognized by α-YchF antibodies (*) in the absence of DTT. (B) Purified YchF was separated on SDS-PAGE under nonreducing conditions as in A (1st dim.), and after Coomassie staining, the YchF monomer and dimer bands were excised out of the gel and separated on a second dimension SDS-PAGE (2nd dim.) under reducing conditions. (C) Purified YchF and lysozyme-lysed E. coli cells expressing YchF were separated under nonreducing conditions on SDS-PAGE and YchF was detected after Western transfer with α-YchF antibodies. (D) Purified wild-type YchF (wt YchF) and a cysteine-free derivative (Cys-free YchF) were reduced and incubated with the thiol-modifying agent, PEG-Mal, and subsequently separated by SDS-PAGE. After Western transfer, the membrane was probed with α-YchF antibodies. (PM)0–6 reflect the number of labeled cysteines. (E, F) Purified Cys-free and wild-type YchF were incubated with the fluorescent thiol-modifying agent fluorescein-5-maleimide. Subsequently, pellet (Pell) and supernatant (Sup) fractions were prepared and samples separated by SDS-PAGE under nonreducing conditions, followed by Coomassie staining (E) or in-gel fluorescence analysis (F).