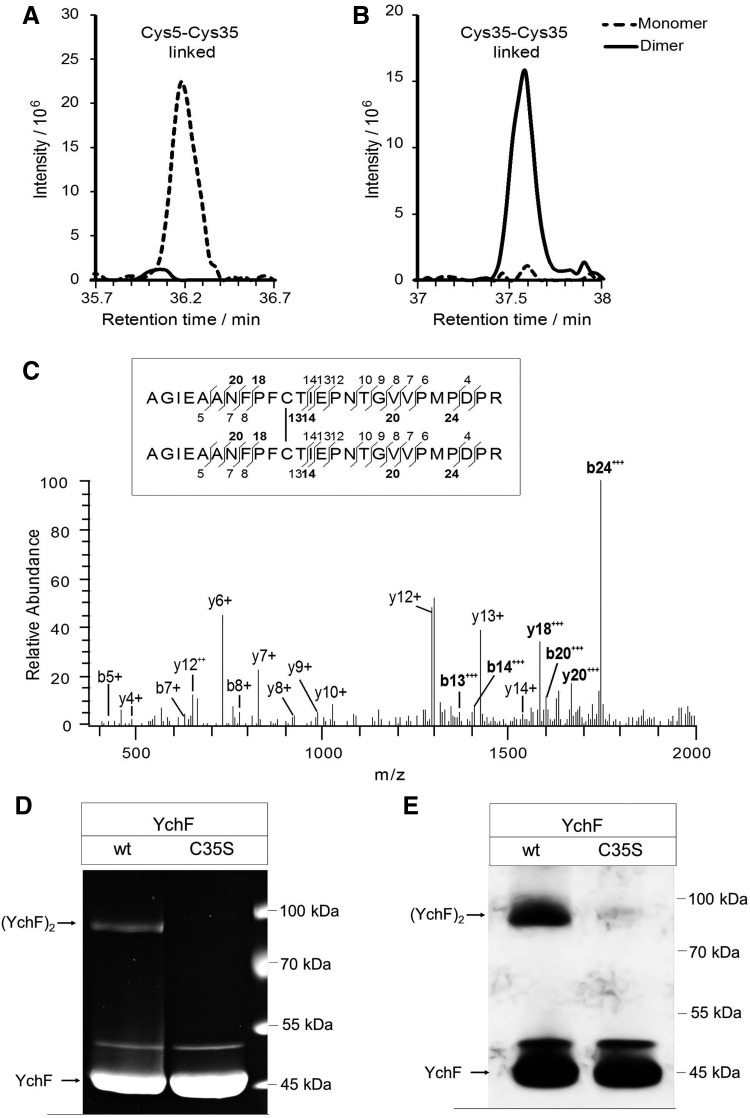
FIG. 3.
Identification and quantification of disulfide-linked peptides in YchF monomer and dimer. (A) Extracted ion chromatograms of the Cys5-Cys35-linked peptides, CGIVGLPNVGK and AGIEAANFPFCTIEPNTGVVPM*PDPR (precursor m/z 953.9757, charge +4), determined by liquid chromatography–mass spectrometry analyses of the monomer (dashed line) and dimer band (solid line). (B) Equivalent to A, but for the Cys35-Cys35-linked peptide AGIEAANFPFCTIEPNTGVVPM*PDPR (precursor m/z 1379.6581, charge +4). (C) Fragment spectrum of the Cys35-Cys35 disulfide-linked peptide AGIEAANFPFCTIEPNTGVVPM*PDPR. b- and y-type fragment ions are annotated in the mass spectrum and in the sequence (inset). Fragments containing the disulfide bridge are shown in bold. (D) Wild-type YchF and YchF containing a single cysteine-to-serine replacement at position 35 (YchFC35S) were purified and separated by SDS-PAGE under nonreducing conditions. Proteins were subsequently stained with Coomassie. (E) As in D, but samples were probed with α-YchF antibodies after Western blotting. M*, oxidized methionine.