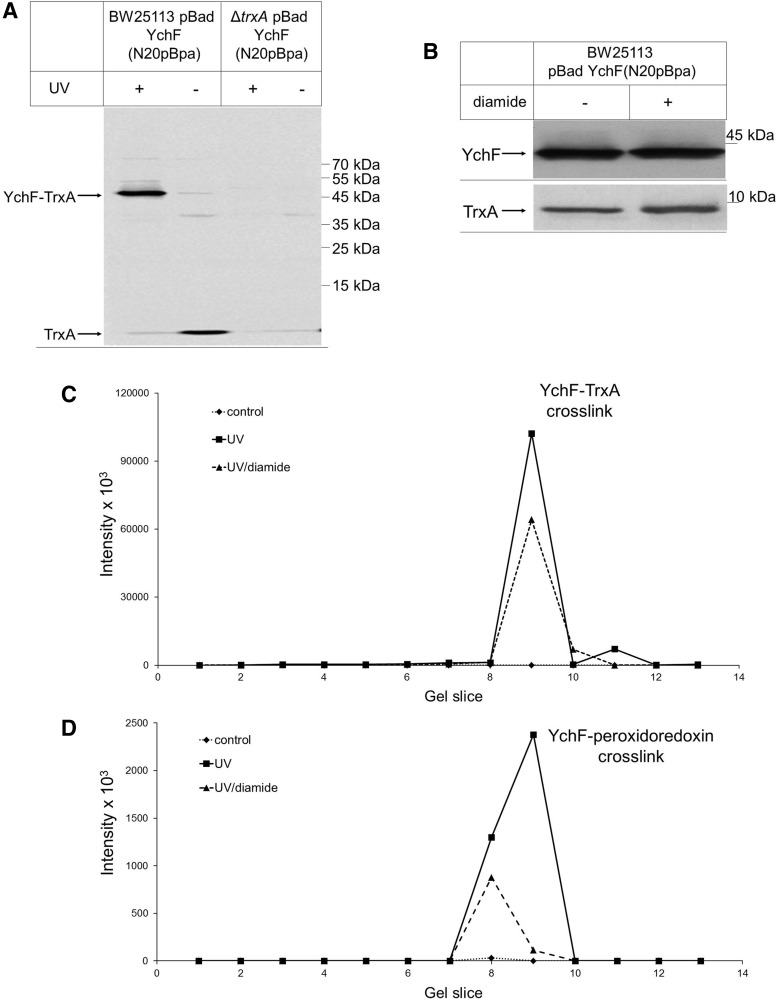
FIG. 5.
YchF cross-links to thioredoxin in vivo. (A) Wild-type E. coli cells or a ΔtrxA E. coli strain expressing YchF with the UV-dependent cross-linker pBpA incorporated at position N20 were either UV exposed (+) or kept in the dark (−). After cross-linking, YchF was purified, separated by SDS-PAGE, and after Western transfer probed with α-TrxA antibodies. (B) Wild-type E. coli cells expressing YchF(N20pBpa) were treated with 50 μM diamide and TCA precipitated. The pellet after centrifugation was separated by SDS-PAGE and after Western transfer probed with either α-YchF or with α-TrxA antibodies, (C, D) YchF(N20pBpa) cross-linking products were separated by SDS-PAGE, individual gel slices corresponding to the MW range of 130 to 40 kDa were excised, subjected to in-gel digestion using trypsin, and analyzed by mass spectrometry. Data were analyzed for peptides derived from TrxA (C) and peroxiredoxin (D). Shown are summed peptide intensities measured in samples obtained without UV exposure (noncross-linked, ♦, dotted line), after UV exposure (cross-linked, ■, solid line), and after UV exposure of diamide-treated cells (▲, dashed line).