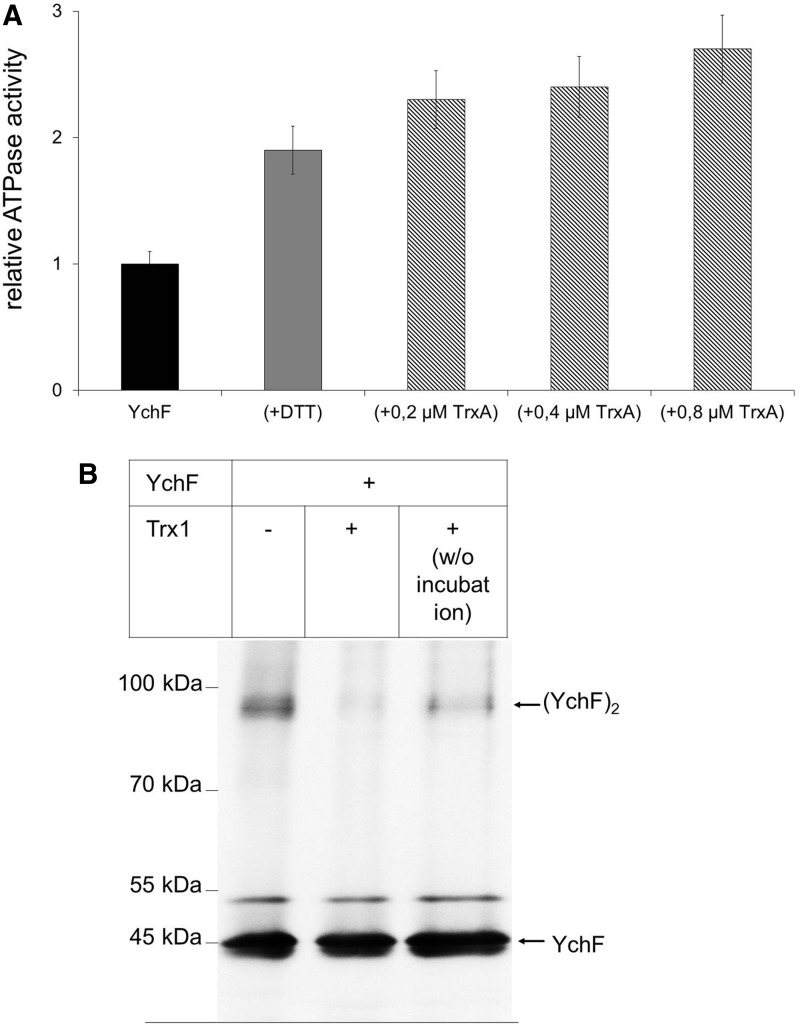
FIG. 7.
Thioredoxin increases the ATPase activity of YchF and reduces the YchF dimer. (A) Purified YchF (0.2 μM) was incubated with either DTT (10 mM) (gray bar) or increasing TrxA concentrations (hatched bars) and the ATPase activity was measured as in Fig. 4. The activity of wild-type YchF was set to one and corresponds to 0.35 nmol ATP/(min × mg protein). The values observed were corrected for the background ATP hydrolysis detected in the purified TrxA sample. (B) Purified YchF was incubated for 30 min at 37°C in the presence or absence of purified TrxA in a 1:1 molar ratio. As a control, TrxA was added to YchF without further incubation (w/o incubation). Samples were analyzed by SDS-PAGE under nonreducing conditions and probed with α-YchF antibodies after Western transfer.