Figure 2.
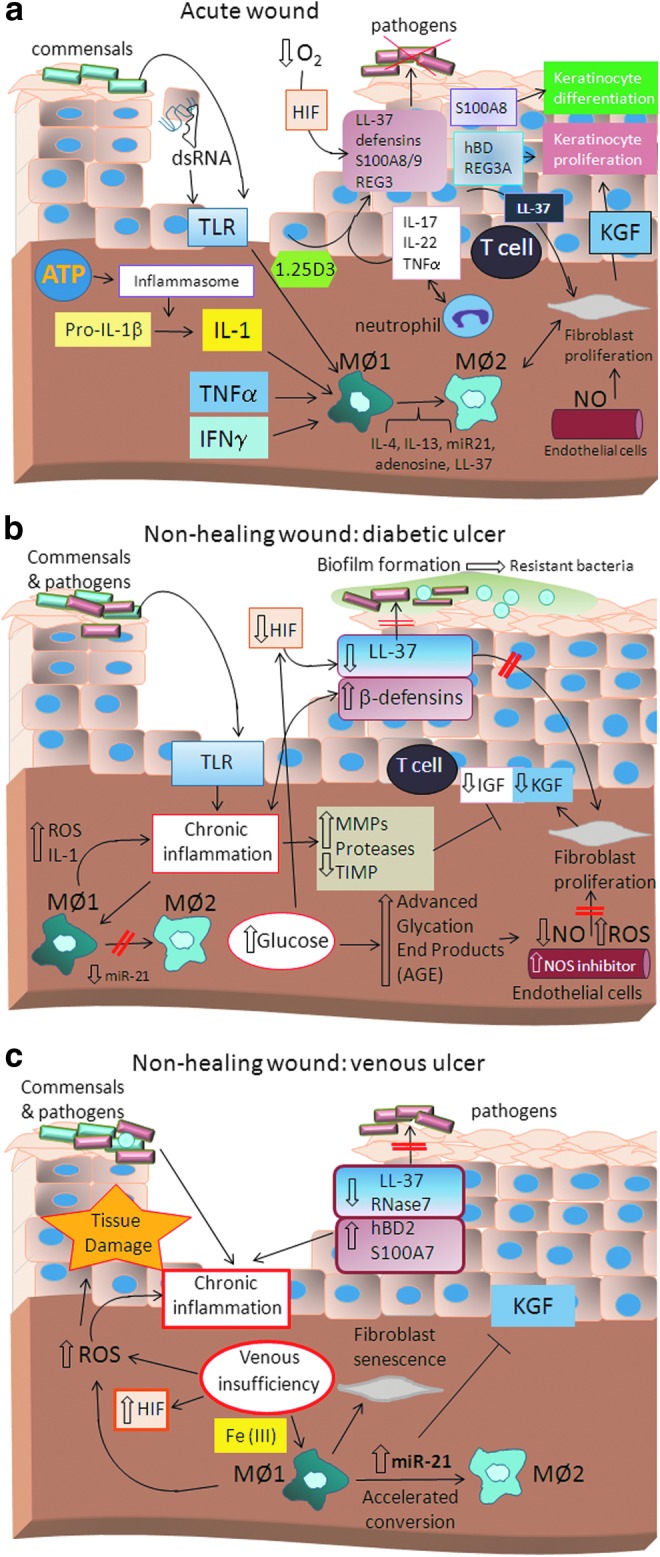
Model of (a) normal wound healing in acute wounds and nonhealing (b) diabetic and (c) venous ulcers. Acute wound healing is characterized by activation of the innate immune system aimed at the successful killing of pathogenic microbes through deployment of antimicrobial effector molecules called AMPs, such as cathelicidin LL-37, β-defensins, and S100 proteins, as well as regenerating islet-derived protein 3 (REG3). Activation of Toll-like receptor (TLR) and other pathogen or danger recognition receptors leads to inflammasome activation and IL-1 production as well as an antimicrobial peptide response. Furthermore, AMP responses are enhanced by low O2 levels in the wound, known to activate HIF1a and through IL-17 and IL-22 produced by resident T cells at the site of injury. IL-1, TLR activation, and interferon (IFN)γ, as well as TNFα drive M1 macrophage differentiation. Following successful decontamination of the wound, the inflammatory phase resolves and the reparative stage, which is characterized by keratinocyte and fibroblast proliferation, begins. The switch from M1 macrophages to M2-like macrophages as well as distinct AMP function is an important regulator of the reparative phase. Wound reepithelialization requires regulated keratinocyte proliferation, migration, and differentiation, which is guided, at least in part, through growth factor production and AMPs. However, under pathogenic conditions, such as diabetic ulcers (b), the normal wound healing response is disturbed. Diabetes is associated with a chronic inflammatory state, which leads ultimately to an imbalance and dysregulation of skin immune function. Dysfunctional AMP, ROS, and protease production result in overt perpetuation of inflammation, tissue destruction, and ineffective decontamination of pathogenic bacteria. High glucose levels are associated with high levels of AGE products ultimately enhancing vascular NO synthase inhibitor, thereby inhibiting NO production. In contrast, venous insufficiency in venous ulcers (c) leads to iron overloading of macrophages, which engulf extravasated erythrocytes. Iron overloading in macrophages has been shown to give rise to M1 macrophages. However, high abundance of miRNA-21 has been associated with accelerated conversion to M2-like macrophages, leading to the paradox that despite increases in inflammatory M1 macrophages, large numbers of M2-like macrophages are generated prematurely. In addition, miRNA-21 inhibits growth factor signaling by yet undefined mechanisms and delays wound closure.
