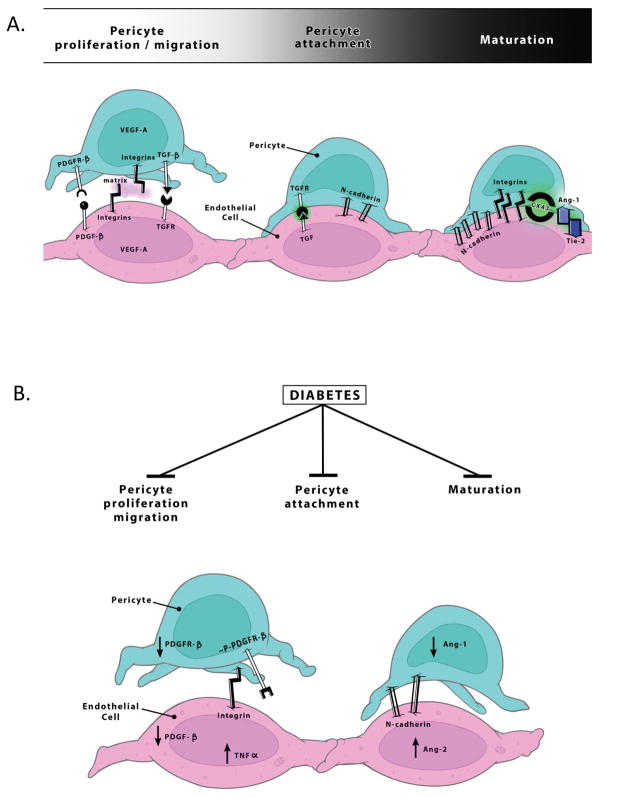
Figure 2. Possible mechanisms contributing to weakened pericyte-endothelial cell interactions in diabetes.
A. At the initiation of angiogenesis, pericytes migrate away from endothelial cells and secrete proangiogenic growth factors like VEGF-A. Pericytes are later recruited in response to PDGF-β secreted by endothelial cells and through the activation of PDGF-R β, they proliferate and enhance the interaction with endothelial cells. Pericytes contribute to the termination of angiogenesis and stabilization of newly formed vessels through barriergenesis. Activation of TGF-β and Ang-1, stimulates expression of N-cadherin, integrins and gap junctions, especially connexin 43 (CX43). As vessel stabilization occurs, pericytes, along with astrocytes, secrete matrix proteins. B. In diabetes, pericyte recruitment, attachment and maturation are impaired. Increased phosphorylated pPDGFR-β, decreased PDGFR-β expression and or altereations in Ang-1 and Ang-2 signaling may contribute to loss of communication between these two cell types.