Significance
Most large mammals are endangered or vulnerable across the globe. Although the loss of charismatic fauna is of great concern, their role in ecosystem function remains poorly characterized. Here, we quantify one potential effect of the decline of large herbivores: the reduction of the greenhouse gas methane released as a byproduct of plant digestion. We examine three time periods where large-scale losses of megaherbivores occurred—the African rinderpest epizootic of the 1890s, the massive Great Plains bison kill-off in the 1860s, and the terminal Pleistocene extinction of megafauna. We find detectable decreases in the global methane budget related to the extirpation of megaherbivores. Our findings underscore the importance of large mammals in regulating ecosystems and feedbacks on climate.
Keywords: trophic downgrading, megaherbivores, rinderpest, bison overkill, Anthropocene
Abstract
Globally, large-bodied wild mammals are in peril. Because “megamammals” have a disproportionate influence on vegetation, trophic interactions, and ecosystem function, declining populations are of considerable conservation concern. However, this is not new; trophic downgrading occurred in the past, including the African rinderpest epizootic of the 1890s, the massive Great Plains bison kill-off in the 1860s, and the terminal Pleistocene extinction of megafauna. Examining the consequences of these earlier events yields insights into contemporary ecosystem function. Here, we focus on changes in methane emissions, produced as a byproduct of enteric fermentation by herbivores. Although methane is ∼200 times less abundant than carbon dioxide in the atmosphere, the greater efficiency of methane in trapping radiation leads to a significant role in radiative forcing of climate. Using global datasets of late Quaternary mammals, domestic livestock, and human population from the United Nations as well as literature sources, we develop a series of allometric regressions relating mammal body mass to population density and CH4 production, which allows estimation of methane production by wild and domestic herbivores for each historic or ancient time period. We find the extirpation of megaherbivores reduced global enteric emissions between 2.2–69.6 Tg CH4 y−1 during the various time periods, representing a decrease of 0.8–34.8% of the overall inputs to tropospheric input. Our analyses suggest that large-bodied mammals have a greater influence on methane emissions than previously appreciated and, further, that changes in the source pool from herbivores can influence global biogeochemical cycles and, potentially, climate.
Recent assessments characterize the conservation status of large-bodied mammals as “precarious” because of a combination of environmental factors and intrinsic traits (1–3). Estimates suggest that 21–36% of all mammals are threatened with extinction (2), with risk especially high for larger animals (1, 2). In Africa, the abundance of large-bodied mammals has decreased by >50% since the 1970s (3); similar declines are reported elsewhere (2). Although the loss of charismatic fauna is of great concern for many reasons, their loss may have unappreciated consequences in terms of ecosystem function.
The decline of large predators and other apex consumers has been connected with the “unraveling” of ecosystems (4). Large-bodied herbivores have a disproportionate impact on vegetation structure and composition (5–7). For example, the transition from the vast “mammoth steppe” of the Pleistocene to the more waterlogged habitats of the Holocene is at least partially owing to the absence of grazing by megaherbivores (5, 7). Certainly, modern studies indicate elephants inhibit woodland regeneration and promote the formation of grasslands; equids play a significant role in the dispersal of large-seeded plants (7, 8). However, as the papers in this Special Feature suggest, megaherbivores influence more than vegetation, including trophic interactions, fire regimes, and other aspects of ecosystem function (9–11).
Large mammals also influence climate. Herbivores release methane as a byproduct of the anaerobic microbial fermentation of plant materials. Although a variety of natural and anthropogenic sources produce methane, for many countries, domestic mammals are the primary source (12). Although ∼200 times less abundant than carbon dioxide in the atmosphere, methane’s greater efficiency in trapping radiation and reactions with other trace gases leads to a significant role in the radiative forcing of climate (12–15). Moreover, CH4 is an attractive and relatively inexpensive target for mitigation because it has a relatively short residence in the atmosphere (14). Although total global emissions are constrained reasonably well (∼500–600 Tg CH4 y−1), quantification by source sector has proven more difficult, with estimates varying by a factor of 2–3 (12, 15). Thus, better characterization is of considerable scientific and policy interest. Although domestic animals are incorporated in CH4 inventories, wildlife are generally considered negligible (e.g., table 7.6 in ref. 12).
Here, we explore the role of wild mammalian herbivores on the global cycling of methane. We use a historical perspective by investigating the influence of the extirpation or reduction of large herbivores on biogeochemistry at three times in the past: (i) the 1890s, when a disastrous rinderpest epizootic wiped out tens of millions of domestic and wild Artiodactyla in Africa; (ii) the mid-1800s, when >30 million bison were killed in the prairies of North America; and (iii) the terminal Pleistocene megafauna extinction (∼13–11.5 ka), when >200 species of large-bodied mammals went extinct globally. Our approach is conceptually simple but computationally more difficult; using a large spatially explicit database of late Quaternary mammals and body mass, we use allometric regressions relating population density, geographic range, and methane output to mass. We also develop empirically based equations relating livestock numbers to human population (Table 1), which allow assessment of the relative importance of enteric emissions from domestic versus wild mammals at various historic times. Our calculations of tropospheric inputs from herbivores are compared with methane budgets derived from ice cores and other sources.
Table 1.
Computed global population size and methane production by domestic livestock from 1800 to 2006
| Species | Equation,* log–log | R2 | Population estimate from regression, ×106 | FAO,* ×106 | Computed methane output, Tg y−1† | ||||||
| 1800 | 1850 | 1890 | 2006 | LP | 1800 | 1850 | 1890 | 2006 | |||
| Cattle | y = 0.718x + 2.165 | 0.98 | 418 | 503 | 609 | 1,409 | 0 | 22.52 | 27.04 | 32.78 | 75.81 |
| Buffalo | y = 0.846x − 0.071 | 0.97 | 34 | 42 | 53 | 180 | 0 | 2.27 | 2.82 | 3.54 | 12.07 |
| Pig | y = 1.717x − 7.662 | 0.97 | 59 | 92 | 145 | 904 | 0 | 1.71 | 2.66 | 4.21 | 26.19 |
| Sheep | y = 0.584x + 3.456 | 0.98 | 460 | 471 | 484 | 1,123 | 0 | 3.44 | 3.75 | 3.85 | 8.94 |
| Goats | y = 0.668x + 2.197 | 0.86 | 161 | 190 | 228 | 893 | 0 | 0.95 | 1.12 | 1.34 | 5.27 |
Enteric emissions computed using an average body mass for each domestic livestock type (SI Appendix, Table S1); estimates could be improved if information on body size classes were available. Values do not include manure production. IPCC estimate (12) for domestic animals =∼80 Tg CH4 y−1, based on 1995–2006.
Rinderpest
Rinderpest, or the cattle plague, is a disease of antiquity with epizootics dating as early as 493 BC (16). Before eradication in 2011, outbreaks occurred with some regularity, often accompanying armies (17). The virus followed the Huns when they invaded Europe, for example, resulting in widespread plague throughout the continent (16). The virus also accompanied the Romans, the Danes, the Crusaders, and Peter the Great in wars of conquest. Rinderpest was the “most dreaded bovine plague known, belonging to a select group of notorious infectious agents that have changed the course of history” (ref. 18, p. 1). Natural hosts included domesticated and wild Artiodactyla, such as water buffalo, cattle, eland, giraffe, impala, and wildebeest (19, 20). The virus was highly communicable and in its most severe form, killed >95% of infected animals (16–18). Outbreaks in the eighteenth century reportedly led to the deaths of ∼200 million cattle (20). In the early 1890s, rinderpest was inadvertently introduced to Africa by Italian or British military operations (20, 21). It quickly spread throughout much of the continent, leading to catastrophic mortality of tens of millions of both domestic and wild mammals (20, 21) (Fig. 1A). The course of this epizootic was devastating [e.g., “The disease swept south like a hurricane, leaving nothing but bleaching skeletons to mark its track” (ref. 20, p. 26)]. The almost complete loss of cattle coupled with severe drought led to widespread human famine, with mortality rates of 30% in Ethiopia and 75% for the Maasai people of Kenya and Tanzania (20).
Fig. 1.

Historic photographs illustrating scope of mammal extirpations. (Upper) Field of dead cattle during rinderpest outbreak in South Africa, 1896. (Lower) A massive pile of American bison (Bison bison) skulls ultimately ground for fertilizer, ∼1870. Images courtesy of Wikimedia Commons.
Great Plains Bison Kill-Off
Bison are the largest extant terrestrial herbivore in North America and Europe and the quintessential symbol of the American Great Plains. Preindustrial population estimates range as high as 50–75 million individuals (22–26) when “the moving multitude...darkened the whole plains” (ref. 23, p. 1197); today only ∼500,000 animals remain. The animals’ abrupt decline is associated with a combination of the construction of the railroads across the Great Plains, when train passengers were encouraged to shoot buffalo as they raced alongside, a drought in the 1840s, and concerted efforts by the United States government to remove an important food and culture base for Native Americans (24, 25) (Fig. 1B). There is good empirical evidence that ∼30 million animals were killed by the mid-1870s (22, 25), leading one soldier to write, “Where there were myriads of buffalo the year before, there were now myriads of carcasses. The air was foul with a sickening stench, and the vast plain, which only a short twelvemonth before teemed with animal life, was a dead, solitary, putrid desert” (ref. 26, p. 133).
Late Pleistocene Megafauna Extinction
At the late Pleistocene (LP) (∼13.5 ka), the mammal assemblage of the New World was as rich as that of modern Africa (27) and included large-bodied animals such as mammoth, horses, camels, llamas, saber-tooth cats, and short-faced bear (28). Within a short time window (29), >150 species were lost in the Americas (27, 28), including all mammals over 600 kg (27). Similar size-biased extinctions occurred in Eurasia and Africa, although far fewer species were affected (∼12 per continent). The extinction was consistently and strikingly size-selective (27), with an average mass of extirpated mammals 858, 973, 970, and 1332 kg for North America, South America, Africa, and Eurasia, respectively (30). For decades, scientists have hotly debated the cause of the extinction (28, 29, 31). Although the issue remains somewhat contentious, many now agree humans played a role through a combination of hunting and habitat alteration (32). However, what has been largely overlooked are the consequences of the global loss of ∼1 billion large-bodied animals on terrestrial ecosystems. Although an earlier effort examined the influence of the LP megafauna extinction on methane emissions in the Americas (10), the examination likely underestimated tropospheric input because it did not include mammals extirpated elsewhere and did not evaluate input from both surviving and extirpated herbivores.
Results and Discussion
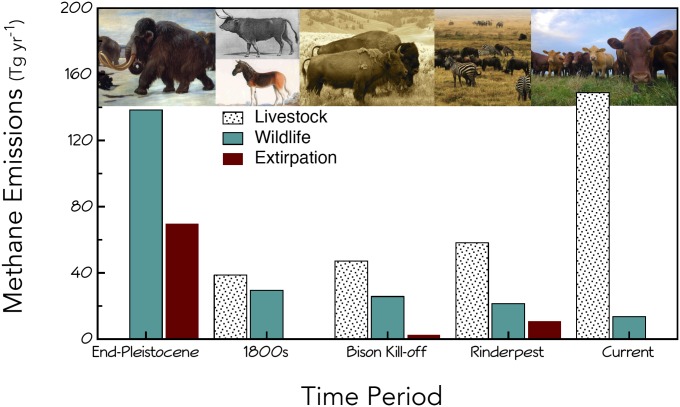
Our computations provide a first-order approximation of the importance of wild mammals in global atmospheric CH4 inventories. We calculate that over time, wildlife have contributed from 13.0 to 138.5 Tg CH4 y−1: values that represent a substantial input to the global atmospheric budget (Tables 1 and 2 and Fig. 2). Indeed, until the 1900s, wildlife enteric emissions were similar or greater than all other natural sources except wetlands (12). Although LP wildlife emissions were similar to that of modern domestic livestock (138.5 versus 147.5 Tg CH4 y−1), between 1800 and 1850, continued urbanization and the rapid growth of the livestock sector led to major changes in the relative proportion of enteric emissions by wild and domestic herbivores (Tables 1 and 2 and Fig. 2). Although the importance of wildlife has continued to decline as habitats around the globe became urbanized (33) (Tables 1 and 2), wildlife currently contribute at least 13.0 Tg CH4 y−1 to the global source pool. Methane inventories generally overlook this important sector because of early work by Crutzen et al. (34), who estimated inputs from wildlife at no more than 2–6 Tg CH4 y−1. However, the authors’ computations greatly underestimated per capita enteric emissions by wild mammals (35). Moreover, recent work suggests that inventories also underestimate the contribution of domestic mammals by a factor of 1.5–1.7 (14), perhaps because of flaws in Intergovernmental Panel on Climate Change (IPCC) methodologies, which only perform well over a fairly narrow range of animal body mass (35). Our results suggest modern enteric emissions by domestic and wild mammals total ∼160 Tg CH4 y−1 (Table 2), substantially more than the ∼90–100 Tg CH4 y−1 currently in IPCC inventories (12).
Table 2.
Calculated enteric emissions by wild and domestic mammals over various historic and ancient time periods
| Event | Time period duration of event (y) | Extent of urbanization* | Total mammal CH4 emission/† with waste, Tg y−1 | Wild mammal CH4 emissions/with waste, Tg y−1 | CH4 reduction/with waste (Tg y−1) | Global‡ CH4, ppbV | Estimated total tropospheric CH4 input§, Tg y−1 | Enteric production as % of total CH4 input | CH4 reduction as % of total mammal input | CH4 reduction as % of total global input |
| Modern | 2006 | 55.5 | 139.6/160.5 | 11.3/13.0 | NA | ∼1,800 | 503–610 | 26.3–31.9 | NA | NA |
| African rinderpest epizootic | 1890s (<10) | 29.8 | 64.9/74.6 | 17.6/20.2 | 9.0/10.4 | 857 | 280–290 | 25.7–26.6 | 13.9 | 3.6–3.7 |
| Great Plains bison kill | 1860s (∼20) | 22.2 | 58.3/67.1 | 20.1/23.1 | 2.0/2.2 | 780–805 | 250–270 | 24.9–26.8 | 3.3 | 0.8–0.9 |
| Preindustrial period | 1800 | 10.8 | 54.4/62.6 | 23.0/26.4 | NA | 741 | 250 | 25.0 | NA | NA |
| LP megafauna extinction (posthuman density estimate) | ∼13.5–11.5 ybp (1.5–2 ka) | 0 | 50.2/57.7 | 50.2/57.7 | 26.1/30.0 | 458–703 | 200–250 | 23.1–28.9 | 52.0 | 12.0–15.0 |
| LP megafauna extinction (prehuman density estimate)¶ | ∼13.5–11.5 ybp (1.5–2 ka) | 0 | 120.4/138.5 | 120.4/138.5 | 60.5/69.6 | 458–703 | 200–250 | 55.4–69.3 | 50.3 | 27.8–34.8 |
NA, not available.
Population estimates for wild mammals reduced by percentage urbanization of natural habitats (33).
Includes values for domestic (cattle, sheep, pigs, buffalo, goats) and wild mammals; we add 15% for waste.
Using an upper 75% quantile regression to offset the additional influence of humans on wildlife population densities (e.g., grazing in natural habitats, etc.) (SI Appendix).
Fig. 2.
Enteric methane emissions by wild (teal) and domestic (spotted) herbivores. The reduction in CH4 emissions resulting from extinctions or extirpation of animals is indicated in red. For the rinderpest epizootic, both domestic and wild animal sectors were affected. Emissions by domestic animals outpaced wild mammals just before 1800 AD. Contributions by wildlife may be slightly underestimated for 1800 and the 1860s because the density equation used was posthuman impact; we would expect the summed contribution of wildlife and domestic livestock to be approximately constant until the modern era when increased crop yield has led to higher production.
We find the extirpation of wildlife in the historic and ancient past had sizeable effects on tropospheric input (Figs. 2 and 3 and Table 2). The 2.2–69.6 Tg CH4 y−1 reduction represents from 3.3% to 50.3% of the mammal source pool and leads to 0.8–34.8% decline in global CH4 input. Our computations are based on a series of highly significant, empirically derived equations, each well constrained with high predictive power. We note our approach was only possible because of extensive global databases constructed in the last decade, which provide data on body mass, trophic affiliation, life history, and geographic distribution of both extinct and extant mammals (30). Here, we limited our computations to the major continents and excluded mammals smaller than 5 kg, although a number of lagomorphs and other small mammals also produce methane (10). Better constraints on error sources would more tightly bracket our estimates but are only likely to strengthen our conclusions.
Fig. 3.
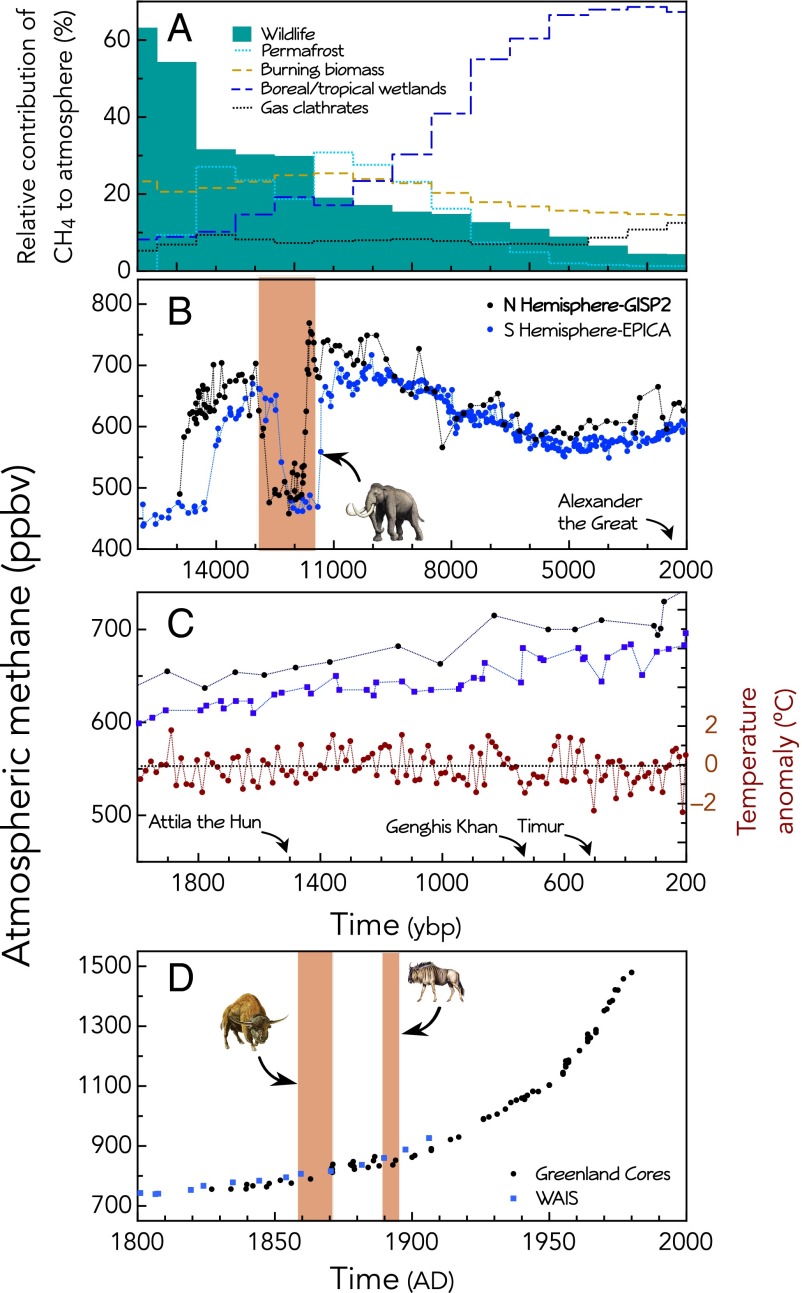
Methane concentrations over time. (A) Modeled calculation of the relative contribution by each source to atmospheric CH4 based on isotopic signatures from Zimov and Zimov (36); we plot the midpoint of the duration of the signal. (B) Atmospheric CH4 concentration from 16 to 2 ka from GISP2 and EPICA Dome C ice cores (47, 48). (C) Atmospheric CH4 concentration from 2 ka to 200 y (same ice cores as B). (D) Atmospheric CH4 concentration over the past 200 y (data sources, refs. 38 and 49–52). Red shading indicates approximate timing of extirpation or extinction event; notable conquests are indicated.
The biggest source of uncertainty in our analysis was population size. The allometric regression we used to relate population density to body size had excellent predictive power for modern ecosystems but was derived using animal populations that had already experienced considerable anthropogenic change. It is likely that before human impact, animal population densities were higher (33, 35, 36). This problem is particularly acute for the LP, when human populations were low. Thus, we modified our estimate of population density for the LP by using an upper quantile regression on the original data used by Damuth (37) (SI Appendix). Although this relationship still includes posthuman effects, it gives more weight to those ecosystems with higher densities and presumably lower anthropogenic impacts. The corrected LP CH4 emissions (Table 2) are consistent with other studies. For example, using the isotopic signature of CH4 from ice cores and computations based on primary productivity, Zimov and Zimov (36) estimated emissions of 90–170 Tg CH4 y−1 by wild mammals in the terminal Pleistocene. Our computations suggest a value of 138.5 Tg CH4 y−1, including manure production (Table 2), about the middle of this range. Furthermore, top-down analysis of the isotopic signature of methane derived from cores suggests that the herbivore sector contributed ∼63–64% of the global input at the LP (36); whereas our posthuman estimate averaged 26%, our revised calculation suggests an average input of 62% (range, 55.4–69.3%; Table 2).
The African rinderpest epizootic (Fig. 3A) profoundly affected both domestic and wild animals, leading to the loss of ∼83 and 438 million animals, respectively. Enteric emissions were reduced by 10.4 Tg CH4 y−1, representing ∼13.9% of the total mammal source pool in the 1890s (Fig. 2 and Table 2). Because herbivores varied in their susceptibility, we weighed mortality probabilities by species vulnerability (19). A lack of data precluded characterization of other epizootics, but they likely also had major impacts on enteric methane emission (Fig. 3 C and D). A recent high-resolution record of atmospheric methane concentration concluded that variation over the late preindustrial Holocene (i.e., the last 1,000 y) might be directly related to times of war and human plague (38). Although the authors concluded that this was related to decreased human population, it is quite possible that reductions were attributable to reduced livestock numbers; outbreaks of rinderpest have followed invading armies throughout much of human history (17). Thus, the long-term cumulative impact of rinderpest may be considerable. Whether the 1890s rinderpest epizootic led to detectable changes in atmospheric CH4 concentrations (Fig. 3D) is difficult to assess because of the paucity of data at this time; widespread measurement networks were only initiated in the 1970s.
The near extirpation of bison is often considered the archetype of human-driven environmental alteration, but the event was fairly minor relative to the rinderpest or terminal Pleistocene (Fig. 2). The slaughter of 30 million bison in the Great Plains in the 1860–1870s led to a reduction of 2.2 Tg CH4 y−1, which represented about 3.3% of the total mammal source pool. Our computation yields values similar to those derived by Kelliher and Clark (39), who used IPCC Tier 2 methodology. The close correspondence is fortuitous; the IPCC Tier 2 only performs well at body masses of 400–800 kg [∼7.4% predictive error (40)], which is about the size of most bison.
Both the rinderpest epizootic and the Bison kill-off were dwarfed by the extent of the LP extinction. More than 200 large-bodied species became extinct (27, 28) in a geological ‘instant’ (29). In the Pleistocene, wild herbivores were the sole source of enteric emissions and a major contributor to the global methane burden (36). Note that we estimate a mean of 62% (range 55.4–69.3; Table 2) as the contribution of enteric production to the overall methane inputs at this time; a value virtually identical to that derived independently from isotopic analysis (Fig. 3A and ref. 36). Because atmospheric concentrations were lower at the LP (∼450–700 vs. ∼1800 ppbV CH4 today), the impact of the loss in emissions was greater (Fig. 2). Our computations suggest the extinction of >1 billion megaherbivores led to a reduction of 69.6 Tg CH4 y−1, about 50% of the overall mammal source pool. The enormity of this event was driven by several factors. First, the mammals extirpated were massive; globally the average was ∼1 ton (30). Because enteric emissions scale nonlinearly, larger herbivores produce more CH4 for their body mass than do smaller ones; a consequence of the disproportionate methanogen growth owing to longer residence times (10). Second, this event was of considerably longer duration (∼1.5–2 ka vs. ∼10–20 y) and much greater spatial extent than either the rinderpest epizootic, whose influence was restricted to the continent of Africa, or the Great Plains Bison kill-off. Finally, we note that our results yield a much larger estimate of the magnitude of the event than earlier work (10) because of the inclusion of extinctions from Africa and Eurasia, use of better constrained scaling relationships, and consideration of manure production.
An interesting question is whether any of these events—rinderpest, the bison kill-off, or the terminal Pleistocene megafauna extinction—led to detectable changes in the atmospheric concentration of methane. Unfortunately, the CH4 record is not sufficiently fine-grained to examine historical events (Fig. 3 C and D), which were of short duration. However, ice cores do indicate an abrupt drop in methane concentrations immediately following the megafauna extinction (Fig. 3B and ref. 15). Moreover, the isotope signature of CH4 transitions abruptly at this time, from one largely produced by mammals to a system dominated by boreal and tropical wetlands (Fig. 3A and ref. 41).
Could the megafauna extinction and corresponding decrease in atmospheric methane have contributed in some way to the abrupt change in climate at the Younger Dryas (YD)? One theory for the YD cold episode at 12.8–11.5 ka is that deglaciation input fresh water to the Atlantic and impacted thermohaline circulation (42). Timing of the ocean mixing effects is difficult to determine, so the explanation remains tentative. Although the importance of methane as a greenhouse gas is undisputed (12), net radiative forcing from the 69.6 Tg CH4 y−1 decrease estimated here is unclear. Methane has fluctuated tremendously over the late Quaternary in response to many factors and not always in concert with temperature; the dynamics of methane are notoriously hard to model (13, 43, 44). Methane is often cited among triggers or additive factors in climate fluctuation (13, 43), but lead–lag relationships are unclear (44), and a general decoupling has been demonstrated within the Northern Hemisphere during the Holocene (44). A further complication is that our analyses suggest the decrease in global methane concentration at the YD was unique; comparison with other drops over the past 800 ka demonstrate that it was significantly more abrupt than others over the late Quaternary (SI Appendix, Table S3 and Figs. S1–S4). The 7–10× faster rate of change suggests additional contributors.
Using a simple atmospheric budgeting approach, we estimated the LP megafauna extinction may have led to a global decrease in temperature ranging from 0.08 to 0.20 °C (SI Appendix, SI Materials and Methods); regional values may have been considerably higher, particularly in northern latitudes. When coupled with other indirect effects of the extinction, such as the reported 0.2 °C decrease in global temperature resulting from changes in surface albedo associated with mammoth-mediated vegetation changes (11), the total effect may approach an upper limit of 0.5 °C. Thus, the integrated effects of the LP extinction approximate the magnitude of anthropogenic climate change over the last century. Thus, we conclude that although the megafauna extinction may well have contributed to the rapidity and severity of the YD, it is clear that other factors were also involved.
Our work demonstrates that human activities have many unintended and unexpected consequences that lead to long-term impacts on the ecosphere. This conclusion is not novel; it is well documented that humans directly and indirectly impact the world’s flora and fauna (1–3). However, the indirect effects of human activities on global biogeochemical cycles are poorly understood. Here, we show that human military operations, which involved the unknowing transport of infected livestock into Africa (20, 21), US government actions to control fractious Native Americans (24, 25), and hunting/habitat alterations by humans invading a new continent (28) had far-reaching repercussions. Each of these events had a measurable impact on the global methane burden (Fig. 2 and Table 2), and the largest, the end Pleistocene extinction, may well have influenced global climate. Thus, we conclude the Anthropocene predates the development of agriculture, complex civilizations, and the industrial age.
Our quantitative approach yields unique insights into the role of wild mammals on global biogeochemical cycles over the historic and ancient past. Moreover, we demonstrate that wild mammals are a significant source pool of enteric emissions and should be included in IPCC inventories. Finally, we note that enteric greenhouse emissions are just one aspect of the megaherbivore influence on biogeochemical cycling. In the absence of heavy grazing by animals, water tables can rise, leading to a slowdown in the rate of nutrient breakdown and recycling, an increase in organic matter accumulation, and a decrease in soil fertility. Selective foraging can also lead to shifts in vegetation structure and composition, which in turn can influence the albedo of the landscape changing heat absorption and reflectivity (11). A synoptic examination of the overall role of megaherbivores in influencing biogeochemical cycling and climate is clearly needed.
Methods
We followed the general approach of Smith et al. (10) but used improved scaling relationships and characterized both wild and domestic herbivore sectors. Using a series of scaling equations relating enteric CH4 production, population, and average adult mass of mammalian herbivores, we calculated total CH4 production at 11.5 ka and 1800, 1850, 1890, and 2006 AD and the reduction caused by extirpations (SI Appendix, Table S1). Values were compared with the global atmospheric methane burden at the time. Species lists and mass (weight in kg) were extracted from the Macroecological Database of Mammalian Body Mass (MOM version 4.1) (30). Analysis was limited to major continents and species >5 kg. For each species (h), we estimated enteric CH4 production (P, in kg CH4⋅y−1) as the product of the species-specific methane emission (M, in kg CH4⋅N-1⋅y−1), animal density (D, in N⋅km−2), and geographic range (A, in km2). To calculate reduction in emissions by rinderpest, we incorporated a term (v), which reflected livestock or wild mammal vulnerability to the virus (19). Assigned susceptibilities were as follows: very high mortality, 95%; high mortality, 75%; moderate mortality, 50%; low mortality, 25%; and very low mortality, 5% (SI Appendix, Table S1); v = 0 for periods other than 1890. To correct for loss of natural habitats over time, we indexed by the degree of urbanization (33) (ut) for each time period (Table 2). Finally, we indexed our overall estimate to reflect CH4 contribution from waste products (m), which we conservatively put at 15% of animal production (12). We sum over all wild herbivores at each time as follows:
| [1] |
This approach was then used to calculate emissions by extirpated wild mammals.
Enteric CH4 emissions for ruminants were characterized by log M = 0.812⋅log W1.171 − 0.619 (r = 0.97; P < 0.004); log M = 3.278⋅log W0.592 − 4.564 (r = 0.98; P < 0.034) was used for monogastric herbivores (35). These equations have significantly lower predictive error than IPCC Tier 1 and Tier 2 approaches (35). We reduced CH4 emissions by 70% for Macropodidae to reflect their unique digestive microbe fauna. For wild mammals, we computed density using log D = 4.23 – 0.75⋅log W (r = −0.86; P < 0.001) from Damuth (37); for the LP, we also used an upper quantile regression of log D = 4.825 – 0.789⋅log W (r = −0.90; P < 0.0001) to correct for human effects on animal density. For geographic range, we used log A = 0.21⋅logW + 6.01 (r = 0.93; P < 0.01) from Smith et al. (10).
We used a slightly different method to compute domestic enteric CH4 emissions (Table 1). We first characterized the relationship between human and domestic livestock numbers for 1961–1981 (sheep 1961–1969), before world agricultural practices became energy intensive (data are from refs. 45 and 46). We hindcast using human population estimates for 1800, 1850, and 1900 to obtain livestock numbers. Values were indexed to reflect contribution of waste. To calculate emissions by livestock for each time period, we summed:
| [2] |
To calculate the impact of herbivores on the global CH4 budget, we summed overall CH4 production at each time period with and without extirpations and introductions:
| [3] |
Supplementary Material
Acknowledgments
We thank S. Finnegan for providing crucial stimulus to actually pursue the project. This research was originally inspired by National Public Radio’s Wait Wait Don’t Tell Me news program. M.A.B. and M.I.P. were fellows in the Program in Interdisciplinary Biological and Biomedical Sciences at the University of New Mexico, supported by National Institute of Biomedical Imaging and Bioengineering Grant T32EB009414 (to F.A.S. and J. H. Brown, principal investigators); J.I.H. was supported by National Institute of General Medical Sciences Grant K12GM088021. The National Science Foundation provided financial support (Grant BIO-0541625 to F.A.S., principal investigator, and S. K. Lyons and S. K. M. Ernest, co-principal investigators).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. C.E.D. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1502547112/-/DCSupplemental.
References
- 1.Cardillo M, et al. Multiple causes of high extinction risk in large mammal species. Science. 2005;309(5738):1239–1241. doi: 10.1126/science.1116030. [DOI] [PubMed] [Google Scholar]
- 2.Schipper J, et al. The status of the world’s land and marine mammals: Diversity, threat, and knowledge. Science. 2008;322(5899):225–230. doi: 10.1126/science.1165115. [DOI] [PubMed] [Google Scholar]
- 3.Craigie ID, et al. Large mammal population declines in Africa’s protected areas. Biol Conserv. 2010;143:2221–2228. [Google Scholar]
- 4.Estes JA, et al. Trophic downgrading of planet Earth. Science. 2011;333(6040):301–306. doi: 10.1126/science.1205106. [DOI] [PubMed] [Google Scholar]
- 5.Johnson CN. Ecological consequences of Late Quaternary extinctions of megafauna. Proc R Soc B Biol Sci. 2009;276:2509–2519. doi: 10.1098/rspb.2008.1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gill JL, Williams JW, Jackson ST, Lininger KB, Robinson GS. Pleistocene megafaunal collapse, novel plant communities, and enhanced fire regimes in North America. Science. 2009;326(5956):1100–1103. doi: 10.1126/science.1179504. [DOI] [PubMed] [Google Scholar]
- 7.Zimov SA, et al. Steppe-tundra transition: A herbivore-driven biome shift at the end of the Pleistocene. Am Nat. 1995;146:765–794. [Google Scholar]
- 8.Western D, Maitumo D. Woodland loss and restoration in a savanna park: A 20-year experiment. Afr J Ecol. 2004;42:111–121. [Google Scholar]
- 9.Pardi MI, Smith FA. Biotic responses of canids to the terminal Pleistocene megafauna extinction. Ecography. 2015 doi: 10.1111/ecog.01596. [DOI] [Google Scholar]
- 10.Smith FA, Elliott SM, Lyons SK. Methane emissions from extinct megafauna. Nat Geosci. 2010;3:374–375. [Google Scholar]
- 11.Doughty CE, Wolf A, Field CB. Biophysical feedbacks between the Pleistocene megafauna extinction and climate: The first human-induced global warming? Geophys Res Lett. 2010;37:L15703. [Google Scholar]
- 12.Solomon S, et al. Technical summary. In: Solomon S, et al., editors. Climate Change 2007: The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge Univ Press; Cambridge, UK: 2007. [Google Scholar]
- 13.Lelieveld JOS, Crutzen PJ, Dentener FJ. Changing concentration, lifetime and climate forcing of atmospheric methane. Tellus B Chem Phys Meterol. 1998;50:128–150. [Google Scholar]
- 14.Miller SM, et al. Anthropogenic emissions of methane in the United States. Proc Natl Acad Sci USA. 2013;110(50):20018–20022. doi: 10.1073/pnas.1314392110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Manning AC, Nisbet EG, Keeling RF, Liss PS. Greenhouse gases in the Earth system: Setting the agenda to 2030. Philos Trans A Math Phys Eng Sci. 2011;369(1943):1885–1890. doi: 10.1098/rsta.2011.0076. [DOI] [PubMed] [Google Scholar]
- 16.Spinage CA. Cattle plague: A history. Springer; New York: 2003. [Google Scholar]
- 17.Roeder P, Rich K. The Global Effort to Eradicate Rinderpest. Intl Food Policy Res Inst; Washington, DC: 2009. [Google Scholar]
- 18.Scott GR, Provost A. Background Paper Prepared for the Food and Agriculture Organization Expert Consultation on the Strategy for Global Rinderpest Eradication, Rome. Food Agric Organ of the UN; Rome: 1992. Global eradication of rinderpest; p. 109. [Google Scholar]
- 19.Plowright W. Viruses transmissible between wild and domestic animals. In: Smith GR, Hearn JP, editors. Reproduction and Disease in Captive and Wild Animals, Symposia of the Zoological Society of London 60. Clarendon; Oxford: 1988. pp. 175–194. [Google Scholar]
- 20.Phoofolo P. In the Time of Plague: The Basotho and the Rinderpest, 1896-1898. Rhodes Univ; Grahamstown, South Africa: 1999. [Google Scholar]
- 21.Mack R. The great African cattle plague epidemic of the 1890’s. Trop Anim Health Prod. 1970;2:210–219. [Google Scholar]
- 22.Flores D. Bison ecology and bison diplomacy: The southern plains from 1800 to 1850. J Am Hist. 1991;78:465–485. [Google Scholar]
- 23.Lewis M, Clark W. In: The History of the Lewis and Clark Expedition. Coues E, editor. Dover; New York: 1806. 1987 reprint of 1893 edition. [Google Scholar]
- 24.Smits DD. The frontier army and the destruction of the buffalo: 1865-1883. West Hist Q. 1994;25:313–338. [Google Scholar]
- 25.Isenberg AC. The Destruction of the Bison: An Environmental History, 1750–1920. Cambridge Univ Press; Cambridge, UK: 2000. [Google Scholar]
- 26.Dodge RI. The Plains of the Great West and Their Inhabitants: Being a Description of the Plains, Game, Indians, & c. of the Great North American Desert. Archer House; New York: 1959. [Google Scholar]
- 27.Lyons SK, Smith FA, Brown JH. Of mice, mastodons and men: Human-mediated extinctions on four continents. Evol Ecol Res. 2004;6:339–358. [Google Scholar]
- 28.Martin PS, Wright HE. Pleistocene Extinctions: The Search for a Cause. Yale Univ Press; New Haven, CT: 1967. [Google Scholar]
- 29.Faith JT, Surovell TA. Synchronous extinction of North America’s Pleistocene mammals. Proc Natl Acad Sci USA. 2009;106(49):20641–20645. doi: 10.1073/pnas.0908153106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smith FA, et al. Body mass of Late Quaternary Mammals. Ecology. 2003;84:3403. [Google Scholar]
- 31.Grayson DK. Deciphering North American Pleistocene extinctions. J Anthropol Res. 2007;63:185–213. [Google Scholar]
- 32.Koch PL, Barnosky AD. Late Quaternary extinctions: State of the debate. Annu Rev Ecol Evol Syst. 2006;37:215–250. [Google Scholar]
- 33.Ellis EC, et al. Anthropogenic transformation of the biomes, 1700 to 2000: Anthropogenic transformation of the biomes. Glob Ecol Biogeogr. 2010;19:589–606. [Google Scholar]
- 34.Crutzen PJ, Aselmann I, Seiler W. Methane production by domestic animals, wild ruminants, other herbivorous fauna, and humans. Tellus B Chem Phys Meterol. 1986;38:271–284. [Google Scholar]
- 35.Smith FA, Lyons SK, Wagner PJ, Elliott SM. The importance of considering animal body mass in IPCC greenhouse inventories and the underappreciated role of wild herbivores. Glob Change Biol. 2015 doi: 10.1111/gcb.12973. [DOI] [PubMed] [Google Scholar]
- 36.Zimov S, Zimov N. Role of megafauna and frozen soil in the atmospheric CH4 dynamics. PLoS One. 2014;9(4):e93331. doi: 10.1371/journal.pone.0093331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Damuth J. Population density and body size in mammals. Nature. 1981;290:699–700. [Google Scholar]
- 38.Mitchell LE, et al. Multidecadal variability of atmospheric methane, 1000–1800 C.E. J Geophys Res. 2011;116(G2):G02007. [Google Scholar]
- 39.Kelliher FM, Clark H. Methane emissions from bison—An historic herd estimate for the North American Great Plains. Agric Meteorol. 2010;150:473–477. [Google Scholar]
- 40.Ciais P. Carbon and Other Biogeochemical Cycles: Final Draft Underlying Scientific Technical Assessment. IPCC Secr; Geneva: 2013. [Google Scholar]
- 41.Ghosh A, et al. Variations in global methane sources and sinks during 1910–2010. Atmos Chem Phys Discuss. 2014;14:27619–27661. [Google Scholar]
- 42.Broecker WS. Abrupt climate change revisited. Glob Planet Change. 2006;54:211–215. [Google Scholar]
- 43.Thompson AM, Chappellaz JA, Fung IY, Kucsera TL. The atmospheric CH4 increase since the Last Glacial Maximum. Tellus B Chem Phys Meterol. 1993;45:242–257. [Google Scholar]
- 44.Whiticar M, Schaefer H. Constraining past global tropospheric methane budgets with carbon and hydrogen isotope ratios in ice. Philos Trans A Math Phys Eng Sci. 2007;365(1856):1793–1828. doi: 10.1098/rsta.2007.2048. [DOI] [PubMed] [Google Scholar]
- 45.Food and Agriculture Organization of the United Nations 2015 FAOSTAT. Food Agric Organ UN Stat Div. Available at faostat3.fao.org/home/E. Accessed March 3, 2015.
- 46.GEOHIVE 2015 GEOHIVE. GEOHIVE - Population Statistics. Available at www.geohive.com. Accessed March 3, 2015.
- 47.Brook EJ, Sowers T, Orchardo J. Rapid variations in atmospheric methane concentration during the past 110,000 years. Science. 1996;273(5278):1087–1091. doi: 10.1126/science.273.5278.1087. [DOI] [PubMed] [Google Scholar]
- 48.Brook E. 2009 Methane Measurements from the GISP2 and Siple Dome Ice Cores. Available at dx.doi.org/10.7265/N58P5XFZ. Accessed March 3, 2015.
- 49.Etheridge DM, Steele LP, Francey RJ, Beschta RL. Atmospheric methane between 1000 A.D. and present: Evidence of anthropogenic emissions and climatic variability. J Geophys Res. 1998;103(D13):15979–15993. [Google Scholar]
- 50.Jouzel J, et al. Orbital and millennial Antarctic climate variability over the past 800,000 years. Science. 2007;317(5839):793–797. doi: 10.1126/science.1141038. [DOI] [PubMed] [Google Scholar]
- 51.Mitchell L, Brook E, Lee JE, Buizert C, Sowers T. Constraints on the late holocene anthropogenic contribution to the atmospheric methane budget. Science. 2013;342(6161):964–966. doi: 10.1126/science.1238920. [DOI] [PubMed] [Google Scholar]
- 52.Etheridge D, Steele L, Francey R, Langenfelds R. Ice Core, Firn Air and Archived Air Atmospheric Methane Concentration Data Technical Report. NOAA/NGDC Paleoclimatol Program; Boulder, CO: 2002. Accessed March 3, 2015. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.