Significance
A standardized network of wildlife surveys across 166 Amazonian hunted and nonhunted forests, combined with basin-wide spatial modeling of central-place hunting pressure, reveal the degree to which arboreal frugivores have been extirpated by hunters and the spatial extent of overhunting for harvest-sensitive frugivore species across the Brazilian Amazon. Simulations based on data from 2,345 1-ha tree plots inventoried throughout Brazilian Amazonia then show widespread erosion of forest carbon stocks in the world’s largest tropical forest region.
Keywords: tropical forest, hunting, forest carbon, ecosystem services, large frugivores
Abstract
Tropical forests are the global cornerstone of biological diversity, and store 55% of the forest carbon stock globally, yet sustained provisioning of these forest ecosystem services may be threatened by hunting-induced extinctions of plant–animal mutualisms that maintain long-term forest dynamics. Large-bodied Atelinae primates and tapirs in particular offer nonredundant seed-dispersal services for many large-seeded Neotropical tree species, which on average have higher wood density than smaller-seeded and wind-dispersed trees. We used field data and models to project the spatial impact of hunting on large primates by ∼1 million rural households throughout the Brazilian Amazon. We then used a unique baseline dataset on 2,345 1-ha tree plots arrayed across the Brazilian Amazon to model changes in aboveground forest biomass under different scenarios of hunting-induced large-bodied frugivore extirpation. We project that defaunation of the most harvest-sensitive species will lead to losses in aboveground biomass of between 2.5–5.8% on average, with some losses as high as 26.5–37.8%. These findings highlight an urgent need to manage the sustainability of game hunting in both protected and unprotected tropical forests, and place full biodiversity integrity, including populations of large frugivorous vertebrates, firmly in the agenda of reducing emissions from deforestation and forest degradation (REDD+) programs.
Tropical forests worldwide store >460 billion tons of carbon—over half of the total atmospheric storage (1)—and tropical forest conversion and degradation account for as much as 20% of global anthropogenic greenhouse gas emissions (2). Tropical forests are also the most species-rich ecosystems on Earth, yet the role of species interactions in stabilizing tropical forest dynamics and maintaining the flow of natural ecosystem services, including long-term forest carbon pools, remains poorly understood. Over 80–96% of all woody plant species in tropical forests produce vertebrate-dispersed fleshy fruits (3, 4), yet many large-bodied frugivore populations in tropical forest regions have already been severely overhunted (5), resulting in functionally “empty” or “half-empty” forests with subsequent disruptions in seed dispersal mutualisms (6). Indeed, the total forest area degraded by unsustainable hunting in the largest remaining tropical forest regions may exceed the combined extent of deforestation, selective logging, and wildfires (7, 8). Even formally decreed forest reserves in remote areas have succumbed to population declines and local extinctions of large vertebrates (9, 10), yet the consequences of this pervasive defaunation process to the persistence of tropical forest ecosystem services remains poorly explored.
Overhunting can amplify dispersal limitation in many large-seeded plant species relying primarily or exclusively on harvest-sensitive large-bodied frugivores. The causal mechanisms through which hunting leads to altered phytodemographics—recruitment bottlenecks resulting from replacement of seedlings from species dispersed by large frugivores with those dispersed by wind, small birds, and bats—has been established in many parts of the humid tropics (e.g., refs. 3 and 11–16). Because stem wood density is a strong predictor of aboveground forest biomass (AGB) across stands with similar basal areas (17–19), overhunting could eventually lead to reduced forest carbon stocks if nonrandom compositional turnover penalizes large-seeded, heavy-wooded species that are primarily dispersed by megafrugivores susceptible to overhunting, thereby favoring wind-dispersed or small-seeded species associated with lower wood density (20–23), as hypothesized by Brodie and Gibbs (24). Evolutionary selection pressure on wood density, or wood-specific gravity (WSG), operates on a trade-off whereby high-WSG trees can achieve a competitive advantage by supporting crowns with greater lateral spread for canopy space, but fast-growing low-WSG trees can reach the canopy and reproduce more quickly (25). Such a competition-colonization trade-off is related to seed dispersal mode because of the observation that small-seeded, often wind-dispersed, trees that are efficient gap colonizers have lower WSG than those bearing large animal-dispersed seeds experiencing greater dispersal limitation (20, 23). However, the trophic cascade between overhunting and reduced stand-scale carbon storage capacity remains controversial in both disturbed and undisturbed tropical forests because: (i) volumetric compensation by species unaffected by this form of dispersal limitation can have the opposite effect (11, 26), (ii) hunting can suppress both plant mutualists (e.g., effective seed dispersers) and antagonists (e.g., seed predators and seedling herbivores) (11), and (iii) several exceptionally large-seeded, heavy-wooded species may continue to be successfully dispersed by large scatter-hoarding rodents that are able to persist in large tracts of overhunted forests (5, 27; but see refs. 28 and 29).
Amazonian forests store ∼125 Pg C in live biomass, or nearly half of the global terrestrial carbon in tropical forests (30), and contribute with ∼15% of the global terrestrial photosynthesis (31). The forests also sustain the highest diversity of fruiting plants (32, 33) and associated mutualists. Harvest-sensitive large-bodied seed-dispersal agents have been extirpated in most tropical forest areas through the combined effects of overhunting, habitat fragmentation, and wildfires (7, 34). However, the degree to which local extinctions of plant–frugivore interactions will destabilize long-term forest ecosystem services, such as high carbon stocks, is yet to be assessed at large spatial scales. Here we use 166 line-transect surveys throughout the Amazon basin to quantitatively assess the degree to which unregulated subsistence hunting affects a key group of forest frugivores (arboreal primates) throughout lowland Amazonia. Based on a spatially explicit biodemographic model (35, 36), we then predict the spatial footprint of hunting-induced population depletion envelopes for a large primate throughout the Brazilian Amazon. Next, we simulate the impact of large-bodied frugivore extirpation on changes in AGB throughout Amazonian forests based on one of the largest tree plot networks available in the tropics, where 129,720 trees ≥ 100 cm in circumference at breast height (CBH) [or ≥31.8 cm in diameter at breast height (DBH)] were inventoried. These large canopy and emergent trees comprise the most important component of tropical forests in terms of phytomass and carbon storage (37). We further model the geographic variation in stand-scale basal area of the most sensitive morphological guild of fruiting trees that is largely dispersed by a small group of large-bodied frugivores, and explain these changes based on a number of physical and floristic variables across the region. Our approach involves multiscale steps ranging from primate population-density estimates derived from the largest standardized series of line-transect censuses ever conducted in a tropical forest region; mapping of population depletion and extinction envelopes throughout the entire Brazilian Amazon; stand-scale inventories (conducted by Projeto RADAMBRASIL since the early 1970s) of tree species composition and size structure across 2,345 1-ha plots distributed throughout the Brazilian Amazon; to simulations of changes in aboveground biomass and carbon stocks throughout this large tree-plot network. These combined approaches provide spatially explicit projections of how aboveground carbon densities may change in overhunted Amazonian forests (SI Results).
Results
Effects of Hunting.
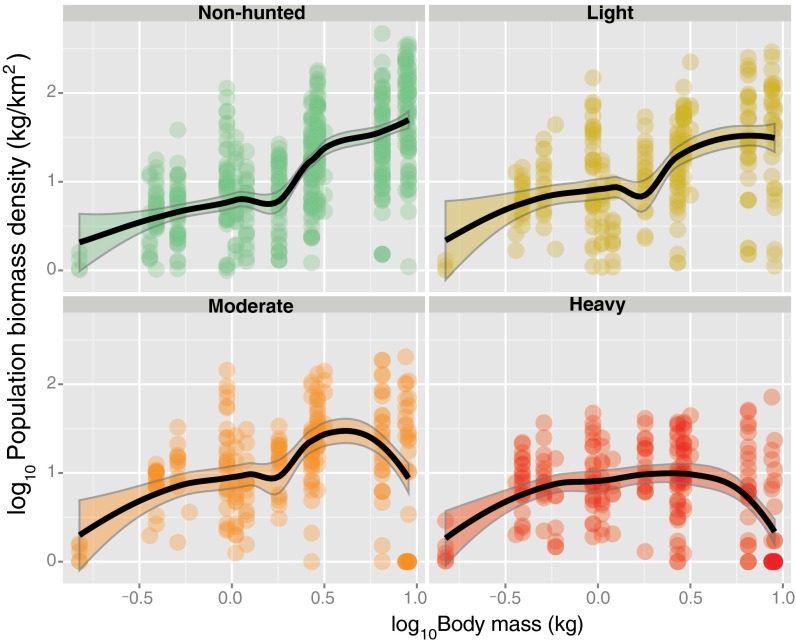
Vertebrate surveys across 166 structurally undisturbed Amazonian forest sites hunted to varying degrees (Fig. S1) indicate that hunting pressure (HP) had a significant effect on overall primate assemblage structure, with the strongest effects on the two large-bodied frugivorous ateline primates (>8.5 kg; spider monkey, Ateles spp. and woolly monkey, Lagothrix spp.). Spider monkey population densities severely declined or were driven to local extinction from an average of 11.14 ± 13.98 ind/km2 (individuals/km2) across 50 nonhunted forest sites to only 0.36 ± 0.81 ind/km2 at 17 heavily hunted sites. The comparable figures for woolly monkeys were 18.97 ± 12.55 ind/km2 at 29 nonhunted sites and 0.79 ± 1.65 ind/km2 at 13 heavily hunted sites. The proportional biomass contribution of these two genera across all 166 forest sites ranged from a mean of 33.8 ± 23.0% for Ateles and 44.9 ± 16.9% for Lagothrix in nonhunted sites to only 2.3 ± 4.5% and 5.5 ± 10.2%, respectively, in persistently hunted sites (Fig. 1 and Fig. S2). These two primate genera had either been completely extirpated in heavily hunted sites or retained population densities of only 0–4% of comparable densities in productivity-equivalent nonhunted sites. This represents a mean loss in population biomass density (and seed-dispersal functions) of 96.97% and 95.80% for spider and woolly monkeys, respectively, across the entire gradient of HP in Amazonian forests.
Fig. 1.
Relationships between primate body mass and population biomass density at 166 Amazonian forest sites surveyed to date, showing the local extirpation or population collapse of large-bodied atelines in heavily hunted sites. All forest sites were hunted to varying degrees but were otherwise structurally undisturbed at the time of line-transect surveys. Data are presented for four major classes of HP (none, light, moderate, and heavy) (SI Results). Black lines represent smoothers within 95% CI regions.
Spatial Extent of Overhunting.
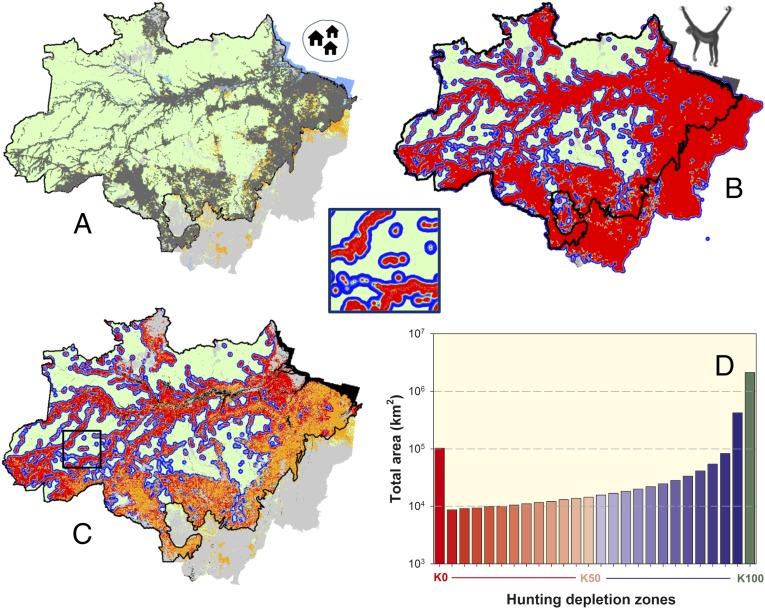
The potential extent of large primate extirpation was predicted by assuming central-place hunting by a single forest hunter for each of the 915,877 georeferenced rural households within the forest phytogeographic boundaries of Brazilian Amazonia (Fig. 2A), which excluded ∼1.38 million mapped rural households in nonforest areas of the political region of Legal Amazonia. Central-place animal protein acquisition, which concentrates hunting effort near households, has the potential to extirpate highly susceptible game species, such as large primates throughout the heavily settled southern and eastern Amazon and along the main tributaries of the Amazon River (Fig. 2 B and C). Nonhunted refugia are, however, maintained within inaccessible regions and large protected areas that are depopulated or sparsely populated. The actual spatial extent of overhunting will vary regionally as a result of local food taboos that may inhibit primate hunting, which are common among recent economic immigrants to the Amazon, or deviations from assumptions of central-place foraging that increase the area accessible to hunters. Nevertheless, the total basin-wide spatial extent of remaining forest areas that likely succumbed to complete extirpation (0.0 K; 103,022 km2) or overhunting (<0.5 K; 236,308 km2) represent 3.3% and 7.5% of the total remaining forest area, respectively, across Brazilian Amazonia. The total area where large primate frugivores have been affected by any level of hunting (<1.0 K) represents 32.4% of all remaining forest areas across the entire Brazilian Amazon (Fig. 2D). This spatial extent (1,017,569 km2) is ∼1.34-times larger than the cumulative area deforested across this region over the 1970–2014 period (www.obt.inpe.br/).
Fig. 2.
Maps of the (A) spatial distribution of all georeferenced rural households across the phytogeographic boundaries of Brazilian Amazonia; (B) population depletion envelopes for a game species that is highly sensitive to hunting (spider monkey, Ateles spp.) based on a biodemographic model that considers both the behavior of central-place hunters and the population dynamics of prey species; and (C) the overall distribution of depletion envelopes excluding all deforested areas as of 2013 (shown in orange). Small Inset square shows depletion envelopes in greater detail. The combined areas across the Brazilian Amazon (D) encompass radially asymmetric annuli around each household in which spider monkeys have been driven to extinction or depleted to small populations <50% of carrying capacity, K (dark to light red annuli) or 50–90% of K (light to dark blue annuli). Green vertical bar represents the total area remaining inaccessible to central-place game hunters.
Correlates of Sites Facing Dispersal Limitation.
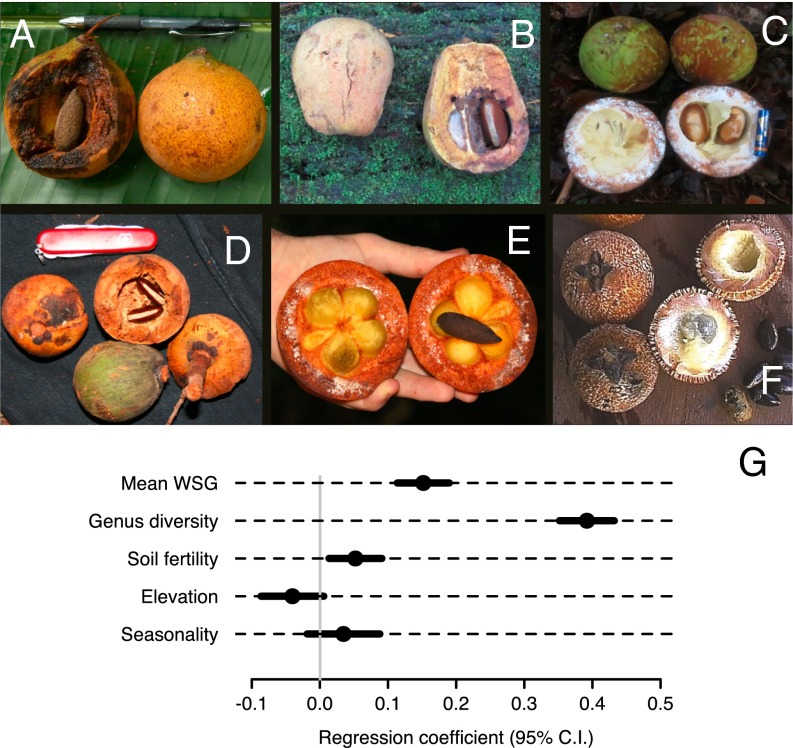
Across the entire network of 2,345 RADAMBRASIL tree plots, each of which containing 31–153 canopy trees, higher plot-scale basal area of trees primarily dispersed by large primates (e.g., Fig. 3) was most strongly associated with high genus-level tree diversity (βstd = 0.39, P < 1−15) and high WSG (βstd = 0.15, P < 2−15) (Fig. 3G). The strong association between high WSG and aggregate basal area of trees primarily dispersed by large primates (Fig. 3G and Table S1) suggests that loss of large-primate dispersed trees would reduce AGB and carbon storage. Soil fertility was also a significant predictor of high basal area of trees at risk for aggravated dispersal limitation (βstd = 0.05, P < 0.01), elevation was a weak and only marginally significant predictor (βstd = –0.04, P = 0.08), and strength of the dry season was not significant (βstd = 0.03, P = 0.19). The autoregressive parameter λ indicated strong and significant spatial autocorrelation across all forest plots (β = 0.69, P < 1e-15), but successfully eliminated spatial autocorrelation of the residuals (Moran’s I, P = 0.58). Aggregate basal area of tree genera susceptible to strong dispersal limitation was also unrelated to contemporary human (household) density (βstd = –0.02, P = 0.32), indicating that settled areas susceptible to overhunting are randomly arrayed relative to floristic mosaics susceptible to AGB loss via this mechanism of dispersal limitation.
Fig. 3.
Examples of whole fresh fruits and seeds within a typical tree family (Sapotaceae) within the morphological guild of undispersed tree species (A–F). These bear one to a few large, gut-dispersed seeds within hard-husked indehiscent exocarps and are most likely to succumb to seedling recruitment bottlenecks resulting from local extinctions of large-bodied prehensile-tailed ateline primates. (G) Coefficient estimates (±95% CI) showing the magnitude and direction of effect sizes of different forest site and biophysical predictors of the aggregate plot-scale basal area of undispersed tree taxa across the entire Brazilian Amazon. For a description of predictor variables, see SI Results.
Effects of Dispersal Bottleneck on Forest AGB.
We simulated large stem turnover in which tree genera predominantly dispersed by large primates (scenario I), or by either large primates or harvest-sensitive lowland tapir (Tapirus spp.; scenario II) remained “undispersed” and replaced by the WSG values of randomly selected cooccurring trees within each of the RADAMBRASIL tree plots (Figs. 3G and 4). These 81 undispersed genera accounted for 28.4% of the taxa but 49.0% of all 129,720 stems across the entire dataset; undispersed genera also had higher mean WSG values than those of dispersed genera (0.6715 vs. 06202 g/cm3) (Fig. S3). Total baseline AGB estimates at time t0 (AGBt0) for canopy trees across all 2,345 plots at the time of RADAMBRASIL forest inventories ranged from 26.08 to 684.39 Mg/ha–1 (mean ± SD, 128.59 ± 57.50 Mg/ha–1), with the highest values concentrated in the northeastern Brazilian Amazon, and declining to the seasonally dry southern fringes of the region (Fig. 5). Postoverhunting estimates of forest biomass at time t1 (AGBt1), however, indicated that 77% and 88% of all plots lost some fraction of their AGB following simulated species turnover under the large-bodied frugivore extirpation scenarios I and II, respectively (Fig. 6). Average AGB losses from t0 to t1 were considerably greater for scenario II (–5.77 ± 5.91%) than for scenario I (–2.53 ± 4.27%), with as much as 26.5% and 37.8% of plot-scale AGB lost under scenarios I and II, respectively. In contrast, null models of stem turnover (with numbers of random tree replacements equivalent to those of scenarios I and II) (SI Results) indicated that there were no net changes in plot-scale AGB (scenarios I: 0.043 ± 0.576%, range = –8.98% to 7.08%; scenario II: 0.062 ± 0.667%, range = –8.53% to 7.91%). Differences in AGB between extirpation and null models (∆agb) considering mean AGB changes over 1,000 simulated tree communities for each plot, were therefore overwhelmingly negative for both scenarios [mean differences for scenario I = –2.57%, 95% confidence interval (CI) = (–2.746, –2.395); paired t tests; t = –28.703, df = 2,344, P < 2.2−16; mean differences for scenario II = –5.83%, (–6.073, –5.587); t = –47.0043, df = 2,344, P value <2.2−16].
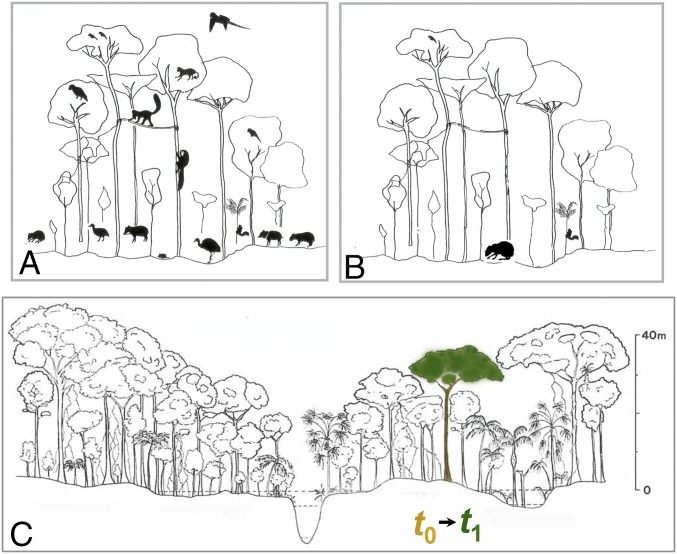
Fig. 4.
Schematic representation of (A) a nonhunted, faunally intact forest and an (B) overhunted, half-empty forest in lowland Amazonia, showing the degree to which large arboreal and terrestrial forest frugivores are either extirpated or severely decline in abundance; and (C) abundance-based lottery transition models considering 129,720 canopy trees contained within 2,345 (1-ha) plots in which undispersed species at time t0 are replaced by any other dispersed species (green tree) at time t1 with a probability that is proportional to their cooccurring abundances at t0 within each plot. A total of 1,000 simulation runs for both random and nonrandom replacements (from t0 to t1) were performed for each plot, with a total number of 48,237 stem substitutions per run.
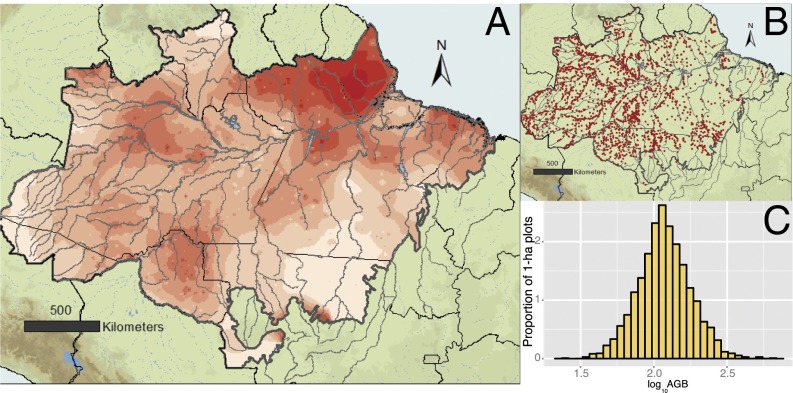
Fig. 5.
(A) Inverse distance-weighting interpolation of forest AGB estimates based on (B) 2,345 1-ha forest plots arrayed across all nine states within the phytogeographic boundaries of Brazilian Amazonia, which were surveyed by the RADAMBRASIL forest inventory program in the 1970s to early 1980s. These estimates are highly variable but follow a (C) log-normal distribution with a mean (±SD) value of 128.6 ± 57.5 Mg/ha‒1, ranging from 26.1 to 684.4 Mg/ha‒1.
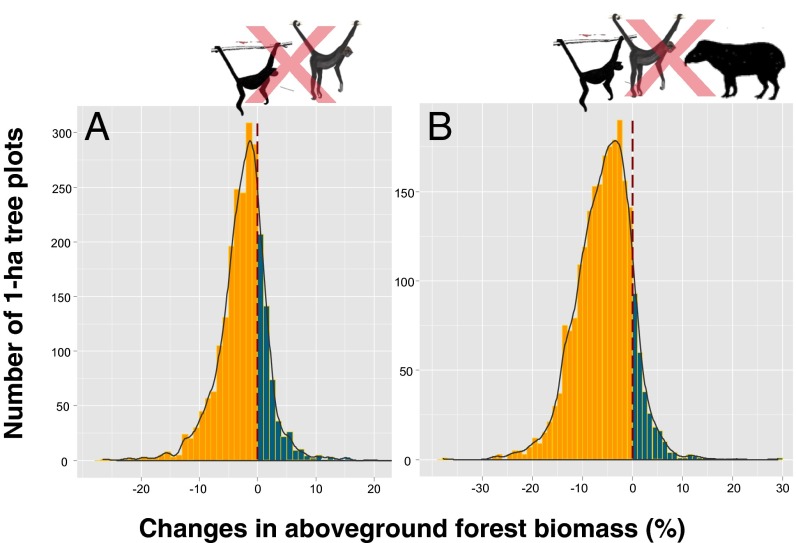
Fig. 6.
Distribution of simulated changes in plot-scale AGB estimates under two mutualism extirpation scenarios considering two functional groups of large-bodied seed dispersal vectors that are highly sensitive to HP throughout Amazonian forests: (A) large atelinae primates (Lagothrix spp. and Ateles spp.), and (B) both large ateline primates and the largest neotropical forest ungulate, lowland tapir (Tapirus terrestris). The resulting ΔAGB values are predominantly negative under both scenarios (orange bars to the left of dashed vertical dashed lines), with 77% and 88% of all 2,345 plots losing biomass under dispersal-limitation scenario I (A) and scenario II (B), respectively.
A total of 420 (17.9%) of the 2,345 RADAMBRASIL plots considered here were surveyed in areas that had already been deforested by 2014 (Fig. 5), which as expected, approximates the proportional area deforested to date across the entire Brazilian Amazon (www.obt.inpe.br/). However, given the variation in composition and abundance of tree functional groups, plots converted to other land-uses—which primarily occur in seasonally dry portions of Amazonia—are expected to lose considerably less AGB and forest carbon than plots in extant forest areas [scenario I: forest plots (–2.578 ± 0.098 SE, n = 1,863); deforested plots (–2.134 ± 0.208 SE, n = 420), t test, P = 0.027; scenario II: forest plots (–5.92 ± 0.13 SE, n = 1,863); deforested plots (–4.59 ± 0.27 SE, n = 420, t test, P < 0.001]. This indicates that remaining forest areas are more vulnerable to the type of dispersal-mediated forest carbon loss documented here (Fig. 7).
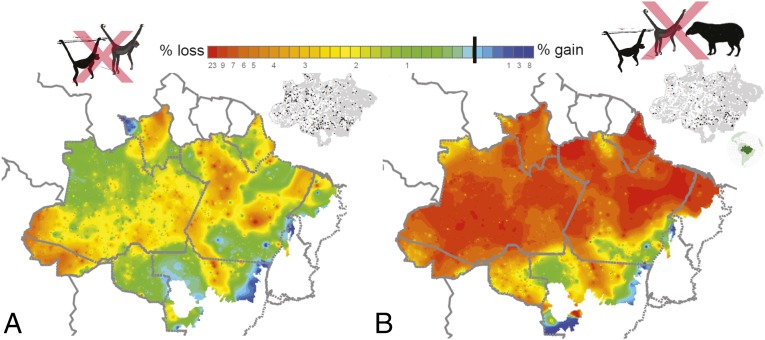
Fig. 7.
Geographic trends in either positive or negative simulated changes in ABG (∆agb) interpolated across the entire Brazilian Amazon from time t0 to t1 considering two conservative faunal extinction scenarios, in which (A) only large ateline primates (i.e., Ateles and Lagothrix) are extirpated (scenario I); and (B) both ateline genera and lowland tapir are extirpated (scenario II). The full spectrum of committed losses or gains in AGB (%) is color-coded from red to blue. Small Inset maps show the total number of 1-ha RADAMBRASIL plots that either lost (white dots) or gained (black dots) biomass across floristic transitions (see SI Results and Fig. 5).
Committed Forest Carbon Loss Estimates.
We extrapolated the basin-wide variation in our estimates of committed net changes in forest carbon stocks per 1-ha plot [mean, 95% CI for scenario I: 3.08 Mg C (2.85–5.93 Mg C); scenario II: 7.36 Mg C (6.97–14.33 Mg C)] to the remaining Brazilian Amazon forest area that had been depleted of critical seed dispersal agents, which excludes all deforested areas and all natural nonforest ecosystems. These estimates now include both all trees ≥31.83 cm DBH sampled by the RADAMBRASIL forest inventory, and the additional AGB contribution from smaller trees between 10 cm and 31.83 cm DBH that we predicted to cooccur in each plot, which on average increased AGB estimates by 94.4% (see SI Results for details). On average, this forest region would be expected to lose 72,869,652 Mg C [95% CI = (67,313,474–78,425,831 Mg C)] and 174,034,150 Mg C (164,710,514–183,357,785 Mg C) under extinction scenarios I and II, respectively, throughout the entire area predicted by our biodemographic model to be heavily overhunted (<0.5 K). However, these estimates would further increase to 313,784,973 Mg C (289,859,441–337,710,505 Mg C) and 749,410,750 Mg C (709,262,120–789,559,381 Mg C) under these scenarios if we consider all forest areas exhibiting any level of hunting-induced declines in the population densities of large-bodied primates (<1.0 K).
Discussion
Although erosion in the full complement of species within a community may affect the functional integrity of ecosystems (38), this relationship remains largely untested in tropical forest systems. Here, we show that: (i) wildlife populations in a small group of large-bodied, harvest-sensitive frugivores have been affected by semisubsistence hunters in as much as 32.4% of all remaining forest cover across the Brazilian Amazon; (ii) that this process is widespread and affects a larger spatial extent than any other pattern of less cryptic and better mapped natural (39) and anthropogenic forest disturbance, including deforestation, logging, and wildfires (7); and (iii) the extirpation of this key group of frugivores can severely degrade the long-term forest dynamics that maintains forest AGB and carbon stocks. Despite the global-scale congruence between carbon stocks and biodiversity value (40), this is not necessarily the case at regional scales (41) so that conservation planning should take into account both the carbon and biodiversity values of remaining tropical forests.
Our results build on plot-scale analyses in Amazonian forests (16, 42), and trait correlations between seed size and wood density (Fig. S4) (20, 23), suggesting that overhunting-induced reductions in seedling recruitment are disproportionately stronger in heavy-wooded tree species. These patterns are corroborated by studies in Mesoamerica (11, 15), Africa (13, 14, 43, 44), and southern Asia (12), showing that severe depletion of large frugivores negatively affects the recruitment, relative abundance, and population growth rate of large-seeded trees. However, our simulations may conservatively exclude some very large-seeded species (typically with seed lengths >45 mm) from our list of candidate taxa projected to undergo severe dispersal limitation, even though these species are often associated with high wood density (Fig. S4). These very large seeds are often primarily scatter-hoarded by large rodents, such as Dasyprocta and Myoprocta (28), rather than ingested and passed intact. We also excluded most medium-seeded species (typically with seed lengths 15–25 mm) that may be dispersed by medium-sized frugivores that often persist in hunted forests (5). Our results are consistent with a Central African study showing that forests that had been both hunted and logged had higher abundances of fast-growing pioneer trees, and supported a biomass 16% lower than that of logged-only forests with similar timber extraction practices (44).
Significant controversy remains over how the Amazonian forest will respond to future scenarios of large-scale human disturbance and climatic change. Some modeling studies have predicted a large-scale dieback of Amazonian forests (45), whereas other predictions show a carbon sink well into the 21st century induced by CO2 fertilization (46, 47). Several studies based on tree-plot networks have shown that tropical forests are either carbon neutral or a carbon sink, with measured positive net carbon sinks in trees ≥10 cm DBH in both Amazonia (0.30% yr–1) (48) and Africa (0.29% yr–1) (49). However, observations based on 321 forest plots show that the Amazon biomass carbon sink over the last two decades may be declining, because of recent leveling of wood productivity increases combined with a sustained long-term increase in tree mortality (47), not least because of severe droughts (44) and wildfires (50). Our analysis reinforces these projections in that patterns of tree replacements, even in structurally intact but overhunted forests, are likely to be dominated by small-seeded, light-wooded, fast-growing, short-lived trees facing little or no dispersal limitation (Fig. S4), which may accelerate overall forest dynamics and reduce carbon stocks. Should these patterns hold over vast tropical forests areas, then even weakly disproportionate recruitment limitation of heavy-wooded tree species in overhunted forests could result in substantial losses of carbon storage over one to a few tree generations.
Our analysis shows that RADAMBRASIL forest plots inventoried in areas of high agricultural value, which were subsequently deforested in the last 35 y, are less sensitive to dispersal-mediated forest carbon loss than more remote forest areas still standing today. Similarly, formerly intact forest areas within the nearly one-fifth of Brazilian Amazonia converted to nonforest land-uses stored a far lower carbon stock per unit area than currently remaining forests (51, 52). This finding suggests that any management intervention either preventing further large-bodied frugivore defaunation or promoting recovery of depleted populations (e.g., through community-based game management programs) would still potentially yield the most benefits in terms of averting future floristic transitions affecting local carbon stocks.
There are a number of additional reasons our estimates of biomass decay and carbon loss from seed-dispersal bottlenecks could be conservative: (i) our plots only take into account trees >31.8 cm DBH, whereas many large-seeded stems that are vulnerable to similar demographic effects are more abundant and below this size cut-off; (ii) our semidefaunated scenarios do not consider the likely infestation of host trees by high-climbing woody lianas, which are typically wind-dispersed, suppress tree growth and survival, often doubling mortality rates, eventually reducing overall forest biomass (53, 54); (iii) our models do not take into account changes in population density of other midsized to large-bodied vertebrate frugivores that gut-disperse large-seeded, heavy-wooded species that also decline in overhunted forests (5); (iv) the strength of seed-dispersal functions may be suppressed nonlinearly and below the density of key dispersal agents in ways that disproportionately reduce seed dispersal effectiveness (55); and (v) dispersal of very-large–seeded species in many overhunted forests is likely suppressed by depleted populations of large scatter-hoarding rodents (28, 29).
The unprecedented recent expansion of Amazonian protected areas, including those strictly protected on paper and extractive reserves, has been extolled as a critical component of global-scale terrestrial carbon reservoirs (56, 57). Indeed, the 610 forest reserves and 2,954 indigenous territories across all nine Amazonian countries may be able to store 11-times more carbon than all unprotected areas combined (58). However, this takes no account of overhunting within protected areas, which is ubiquitous across Amazonia (9), again reinforcing the notion that protecting forests against structural degradation alone is not enough to maintain baseline carbon stocks in perpetuity if megafrugivores are extirpated from otherwise intact forests.
Large-bodied fruit consumers that disperse viable seeds provide a critical ecosystem service maintaining large-seeded, heavy-wooded tree populations, which may have consequences for long-term AGB and carbon storage in tropical forests worldwide. Our population models and empirical data from 166 Amazonian forest sites show that large primates and other harvest-sensitive frugivores (5) can be rapidly extirpated by unregulated hunting and that this process has occurred at vast spatial scales. Although rates of carbon loss per unit area in overhunted forests are more gradual and lower than in deforested areas, the total area impacted by overhunting far exceeds the area affected by either deforestation or structural forest degradation induced by selective logging. We project that long coevolved mutualisms involving large canopy trees and large-bodied frugivores in tropical forests maintain between 158.6 and 378.9 Mg C/km‒2 in aboveground carbon storage, assuming scenarios I and II, respectively. However, these estimates would further increase to between 308.4 and 736.5 Mg C/km‒2 under these scenarios, if we extrapolate plot-scale rates of carbon loss for canopy trees to all coexisting trees above 10-cm DBH predicted to occur in those plots (see SI Results for details). This would represent a current value of between US$ 5.91 and 13.65 trillion if extrapolated to the entire remaining forest cover across the Brazilian Amazon alone based on modest transaction prices (US$ 5/Mg C) in global carbon markets under the reducing emissions from deforestation and forest degradation (REDD+) framework, and these estimates could increase by 20% if carbon losses were extended to belowground biomass (59). Preventing the loss of large-bodied frugivores with effective spatial zoning and wildlife management is likely to contribute to both long-term maintenance of tropical forest carbon stocks, as well as food security for semisubsistence forest dweller that rely on game vertebrates, as long as they are allowed to persist as ecologically viable populations in forest ecosystems containing a full complement of large vertebrate species.
Supplementary Material
Acknowledgments
We thank all field assistants who helped during fieldwork, and T. Gardner and three referees for comments on the paper. Forest vertebrate surveys (1987–2014) throughout the lowland Amazon were funded by the Natural Environment Research Council, the Betty and Gordon Moore Foundation, and Conservation International. C.A.P. and T.L. were supported by grants from Comissao de Aperfeiçoamento de Pessoal de Nival Superior (004/2012) and a National Science Foundation Postdoctoral Research Fellowship in Biology, respectively.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. C.E.D. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1516525113/-/DCSupplemental.
References
- 1.Pan Y, et al. A large and persistent carbon sink in the world’s forests. Science. 2011;333(6045):988–993. doi: 10.1126/science.1201609. [DOI] [PubMed] [Google Scholar]
- 2.Quéré CL, et al. The global carbon budget 1959–2011. Earth System Science Data. 2013;5(1):165–185. [Google Scholar]
- 3.Peres CA, Roosmalen Mv. Patterns of primate frugivory in Amazonia and the Guianan shield: Implications to the demography of large-seeded plants in overhunted tropical forests. In: Levey D, Silva W, Galetti M, editors. Seed Dispersal and Frugivory: Ecology, Evolution and Conservation. CABI International; Oxford: 2002. pp. 407–423. [Google Scholar]
- 4.Gentry AH. Neotropical floristic diversity: Phytogeographical connections between Central and South America, Pleistocene climatic fluctuations, or an accident of the Andean orogeny? Ann Mo Bot Gard. 1982;69(3):557–593. [Google Scholar]
- 5.Peres CA, Palacios E. Basin-wide effects of game harvest on vertebrate population densities in Amazonian forests: Implications for animal-mediated seed dispersal. Biotropica. 2007;39(3):304–315. [Google Scholar]
- 6.Redford KH. The empty forest. Bioscience. 1992;42(6):412–422. [Google Scholar]
- 7.Peres CA, Barlow J, Laurance WF. Detecting anthropogenic disturbance in tropical forests. Trends Ecol Evol. 2006;21(5):227–229. doi: 10.1016/j.tree.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 8.Milner-Gulland EJ, Bennett EL. Wild meat: The bigger picture. Trends Ecol Evol. 2003;18(7):351–357. [Google Scholar]
- 9.Peres CA, Lake IR. Extent of nontimber resource extraction in tropical forests: Accessibility to game vertebrates by hunters in the Amazon basin. Conserv Biol. 2003;17(2):521–535. [Google Scholar]
- 10.Harrison RD. Emptying the forest: Hunting and the extirpation of wildlife from tropical nature reserves. Bioscience. 2011;61(11):919–924. [Google Scholar]
- 11.Wright SJ, et al. The plight of large animals in tropical forests and the consequences for plant regeneration. Biotropica. 2007;39(3):289–291. [Google Scholar]
- 12.Brodie JF, Helmy OE, Brockelman WY, Maron JL. Bushmeat poaching reduces the seed dispersal and population growth rate of a mammal-dispersed tree. Ecol Appl. 2009;19(4):854–863. doi: 10.1890/08-0955.1. [DOI] [PubMed] [Google Scholar]
- 13.Vanthomme H, Bellé B, Forget PM. Bushmeat hunting alters recruitment of large‐seeded plant species in Central Africa. Biotropica. 2010;42(6):672–679. [Google Scholar]
- 14.Effiom EO, Nuñez-Iturri G, Smith HG, Ottosson U, Olsson O. Bushmeat hunting changes regeneration of African rainforests. Proc Biol Sci. 2013;280(1759):20130246. doi: 10.1098/rspb.2013.0246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kurten EL, Wright SJ, Carson WP. Hunting alters seedling functional trait composition in a Neotropical forest. Ecology. 2015;96(7):1923–1932. doi: 10.1890/14-1735.1. [DOI] [PubMed] [Google Scholar]
- 16.Terborgh J, et al. Tree recruitment in an empty forest. Ecology. 2008;89(6):1757–1768. doi: 10.1890/07-0479.1. [DOI] [PubMed] [Google Scholar]
- 17.Bunker DE, et al. Species loss and aboveground carbon storage in a tropical forest. Science. 2005;310(5750):1029–1031. doi: 10.1126/science.1117682. [DOI] [PubMed] [Google Scholar]
- 18.Stegen JC, Swenson NG, Valencia R, Enquist BJ, Thompson J. Above‐ground forest biomass is not consistently related to wood density in tropical forests. Glob Ecol Biogeogr. 2009;18(5):617–625. [Google Scholar]
- 19.Baker T, et al. Do species traits determine patterns of wood production in Amazonian forests? Biogeosciences. 2009;6:297–307. [Google Scholar]
- 20.Queenborough SA, et al. Seed mass, abundance and breeding system among tropical forest species: Do dioecious species exhibit compensatory reproduction or abundances? J Ecol. 2009;97(3):555–566. [Google Scholar]
- 21.ter Steege H, Hammond DS. Character convergence, diversity, and disturbance in tropical rain forest in Guyana. Ecology. 2001;82(11):3197–3212. [Google Scholar]
- 22.Saldana-Acosta A, et al. Variation of functional traits in trees from a biogeographically complex Mexican cloud forest. Acta Oecologica. 2008;34(1):111–121. [Google Scholar]
- 23.Wright IJ, et al. Relationships among ecologically important dimensions of plant trait variation in seven Neotropical forests. Ann Bot (Lond) 2007;99(5):1003–1015. doi: 10.1093/aob/mcl066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brodie JF, Gibbs HK. Bushmeat hunting as climate threat. Science. 2009;326(5951):364–365. doi: 10.1126/science.326_364b. [DOI] [PubMed] [Google Scholar]
- 25.Swenson NG, Enquist BJ. Ecological and evolutionary determinants of a key plant functional trait: Wood density and its community-wide variation across latitude and elevation. Am J Bot. 2007;94(3):451–459. doi: 10.3732/ajb.94.3.451. [DOI] [PubMed] [Google Scholar]
- 26.Jansen PA, et al. Thieving rodents as substitute dispersers of megafaunal seeds. Proc Natl Acad Sci USA. 2012;109(31):12610–12615. doi: 10.1073/pnas.1205184109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peres CA. Synergistic effects of subsistence hunting and habitat fragmentation on Amazonian forest vertebrates. Conserv Biol. 2001;15(6):1490–1505. [Google Scholar]
- 28.Forget PM, Jansen PA. Hunting increases dispersal limitation in the tree Carapa procera, a nontimber forest product. Conserv Biol. 2007;21(1):106–113. doi: 10.1111/j.1523-1739.2006.00590.x. [DOI] [PubMed] [Google Scholar]
- 29.Galetti M, et al. Defaunation affects the populations and diets of rodents in Neotropical rainforests. Biol Conserv. 2015;190:2–7. [Google Scholar]
- 30.Feldpausch TR, et al. Tree height integrated into pantropical forest biomass estimates. Biogeosciences. 2012;8(5):3381–3403. [Google Scholar]
- 31.Field CB, Behrenfeld MJ, Randerson JT, Falkowski P. Primary production of the biosphere: Integrating terrestrial and oceanic components. Science. 1998;281(5374):237–240. doi: 10.1126/science.281.5374.237. [DOI] [PubMed] [Google Scholar]
- 32.ter Steege H, et al. Hyperdominance in the Amazonian tree flora. Science. 2013;342(6156):1243092. doi: 10.1126/science.1243092. [DOI] [PubMed] [Google Scholar]
- 33.van Roosmalen MG. Fruits of the Guianan Flora. Utrecht University, Wageningen Agricultural University; Utrecht, The Netherlands: 1985. [Google Scholar]
- 34.Dirzo R, et al. Defaunation in the Anthropocene. Science. 2014;345(6195):401–406. doi: 10.1126/science.1251817. [DOI] [PubMed] [Google Scholar]
- 35.Levi T, Shepard GH, Ohl-Schacherer J, Peres CA, Yu DW. Modelling the long-term sustainability of indigenous hunting in Manu National Park, Peru: Landscape-scale management implications for Amazonia. J Appl Ecol. 2009;46(4):804–814. [Google Scholar]
- 36.Levi T, et al. Spatial tools for modeling the sustainability of subsistence hunting in tropical forests. Ecol Appl. 2011;21(5):1802–1818. doi: 10.1890/10-0375.1. [DOI] [PubMed] [Google Scholar]
- 37.Stephenson NL, et al. Rate of tree carbon accumulation increases continuously with tree size. Nature. 2014;507(7490):90–93. doi: 10.1038/nature12914. [DOI] [PubMed] [Google Scholar]
- 38.Pasari JR, Levi T, Zavaleta ES, Tilman D. Several scales of biodiversity affect ecosystem multifunctionality. Proc Natl Acad Sci USA. 2013;110(25):10219–10222. doi: 10.1073/pnas.1220333110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Espírito-Santo FD, et al. Size and frequency of natural forest disturbances and the Amazon forest carbon balance. Nat Commun. 2014;5:3434. doi: 10.1038/ncomms4434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Strassburg BB, et al. Global congruence of carbon storage and biodiversity in terrestrial ecosystems. Conserv Lett. 2010;3(2):98–105. [Google Scholar]
- 41.Putz F, Redford K. Dangers of carbon-based conservation. Glob Environ Change. 2009;19(4):400–401. [Google Scholar]
- 42.Nuñez-Iturri G, Howe HF. Bushmeat and the fate of trees with seeds dispersed by large primates in a lowland rain forest in western Amazonia. Biotropica. 2007;39(3):348–354. [Google Scholar]
- 43.Effiom EO, Birkhofer K, Smith HG, Olsson O. Changes of community composition at multiple trophic levels due to hunting in Nigerian tropical forests. Ecography. 2014;37(4):367–377. [Google Scholar]
- 44.Poulsen JR, Clark CJ, Palmer TM. Ecological erosion of an Afrotropical forest and potential consequences for tree recruitment and forest biomass. Biol Conserv. 2013;163:122–130. [Google Scholar]
- 45.Cox PM, Betts RA, Jones CD, Spall SA, Totterdell IJ. Acceleration of global warming due to carbon-cycle feedbacks in a coupled climate model. Nature. 2000;408(6809):184–187. doi: 10.1038/35041539. [DOI] [PubMed] [Google Scholar]
- 46.Huntingford C, et al. Simulated resilience of tropical rainforests to CO2-induced climate change. Nat Geosci. 2013;6(4):268–273. [Google Scholar]
- 47.Brienen RJ, et al. Long-term decline of the Amazon carbon sink. Nature. 2015;519(7543):344–348. doi: 10.1038/nature14283. [DOI] [PubMed] [Google Scholar]
- 48.Phillips OL, et al. Drought sensitivity of the Amazon rainforest. Science. 2009;323(5919):1344–1347. doi: 10.1126/science.1164033. [DOI] [PubMed] [Google Scholar]
- 49.Lewis SL, et al. Increasing carbon storage in intact African tropical forests. Nature. 2009;457(7232):1003–1006. doi: 10.1038/nature07771. [DOI] [PubMed] [Google Scholar]
- 50.Barlow J, Peres CA. Fire-mediated dieback and compositional cascade in an Amazonian forest. Philos Trans R Soc Lond B Biol Sci. 2008;363(1498):1787–1794. doi: 10.1098/rstb.2007.0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Loarie SR, Asner GP, Field CB. Boosted carbon emissions from Amazon deforestation. Geophys Res Lett. 2009;36(14):L14810. [Google Scholar]
- 52.Nogueira EM, Nelson BW, Fearnside PM, França MB, de Oliveira ACA. Tree height in Brazil’s ‘arc of deforestation’: Shorter trees in south and southwest Amazonia imply lower biomass. For Ecol Manage. 2008;255(7):2963–2972. [Google Scholar]
- 53.Wright SJ, Hernandéz A, Condit R. Bushmeat harvest alters seedling banks by favoring lianas, large seeds, and seeds dispersed by bats, birds, and wind. Biotropica. 2007;39:363–371. [Google Scholar]
- 54.van der Heijden GM, Powers JS, Schnitzer SA. Lianas reduce carbon accumulation and storage in tropical forests. Proc Natl Acad Sci USA. 2015;112(43):13267–13271. doi: 10.1073/pnas.1504869112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.McConkey KR, Drake DR. Flying foxes cease to function as seed dispersers long before they become rare. Ecology. 2006;87(2):271–276. doi: 10.1890/05-0386. [DOI] [PubMed] [Google Scholar]
- 56.Ricketts TH, et al. Indigenous lands, protected areas, and slowing climate change. PLoS Biol. 2010;8(3):e1000331. doi: 10.1371/journal.pbio.1000331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Soares-Filho B, et al. Role of Brazilian Amazon protected areas in climate change mitigation. Proc Natl Acad Sci USA. 2010;107(24):10821–10826. doi: 10.1073/pnas.0913048107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Walker W, et al. Forest carbon in Amazonia: The unrecognized contribution of indigenous territories and protected natural areas. Carbon Management. 2015;5(5-6):479–485. [Google Scholar]
- 59.Baccini A, et al. Estimated carbon dioxide emissions from tropical deforestation improved by carbon-density maps. Nat Clim Chang. 2012;2(3):182–185. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.