Abstract
Trophic rewilding is an ecological restoration strategy that uses species introductions to restore top-down trophic interactions and associated trophic cascades to promote self-regulating biodiverse ecosystems. Given the importance of large animals in trophic cascades and their widespread losses and resulting trophic downgrading, it often focuses on restoring functional megafaunas. Trophic rewilding is increasingly being implemented for conservation, but remains controversial. Here, we provide a synthesis of its current scientific basis, highlighting trophic cascades as the key conceptual framework, discussing the main lessons learned from ongoing rewilding projects, systematically reviewing the current literature, and highlighting unintentional rewilding and spontaneous wildlife comebacks as underused sources of information. Together, these lines of evidence show that trophic cascades may be restored via species reintroductions and ecological replacements. It is clear, however, that megafauna effects may be affected by poorly understood trophic complexity effects and interactions with landscape settings, human activities, and other factors. Unfortunately, empirical research on trophic rewilding is still rare, fragmented, and geographically biased, with the literature dominated by essays and opinion pieces. We highlight the need for applied programs to include hypothesis testing and science-based monitoring, and outline priorities for future research, notably assessing the role of trophic complexity, interplay with landscape settings, land use, and climate change, as well as developing the global scope for rewilding and tools to optimize benefits and reduce human–wildlife conflicts. Finally, we recommend developing a decision framework for species selection, building on functional and phylogenetic information and with attention to the potential contribution from synthetic biology.
Keywords: conservation, megafauna, reintroduction, restoration, trophic cascades
Human impacts are so pervasive that a new geological epoch has been proposed: the Anthropocene (1). The effects on ecosystems and biodiversity are one of the biggest challenges facing modern society. Large-bodied animals are particularly affected, with massive prehistoric extinctions (2–4) and severe declines in many extant species (5). Over the last decades it has become increasingly clear that large animals are often important for ecosystem function and biodiversity via trophic cascades, the propagation of consumer impacts downward through food webs (6, 7). Their widespread losses have led to trophic downgrading on a global scale, with negative effects on ecosystems and biodiversity (6–8).
These observations have inspired a new ecological restoration approach that we here refer to as “trophic rewilding.” The rewilding concept was introduced in the late 20th century as a large-scale conservation strategy, focused on establishment of core wilderness areas, enhanced connectivity, and restoration of keystone species (9–11). Subsequently, rewilding has become an increasingly popular term, but with varied meanings (12, 13). We here focus on rewilding as trophic rewilding, defined as species introductions to restore top-down trophic interactions and associated trophic cascades to promote self-regulating biodiverse ecosystems. An important alternative rewilding concept is passive management without any human interference (12, 14, 15). Most or all approaches to rewilding as an ecological restoration method involve restoring natural processes to promote ecosystems that maintain biodiversity with little or no need for ongoing human management. A key development for trophic rewilding has been the proposal for “Pleistocene rewilding,” advocated to restore ecosystem function by rebuilding rich megafaunas (16–18), thereby overcoming the massive prehistoric extinctions linked to Homo sapiens’ global expansion (2, 4). The merits of this pre-Homo sapiens baseline relative to more recent ones have been much discussed (12). A key point in its favor is that rich post-Mesozoic megafaunas evolved by 40 million y ago (19) and have been characteristic of Earth’s ecosystems until the spread of Homo sapiens (20, 21). Hence, most extant species have evolved in megafauna-rich ecosystems and should be adapted to such conditions (22, 23). A further controversial aspect is the proposed use of nonnative species as ecological replacements for globally extinct species (24, 25). However, this also exemplifies that letting pre-Homo sapiens conditions guide rewilding can be done pragmatically. Indeed, it is not only discussed and applied in relation to wild lands, but also human-occupied landscapes (13, 25, 26), aiming to increase the ability of ecosystems to maintain biodiversity with minimal management, but recognizing that some interference may be needed.
We here provide an overview of the scientific basis for trophic rewilding, discussing theory and practical lessons, providing a systematic review of the international scientific literature, and highlighting underused sources of information. We then outline research priorities and finally discuss how to select species for rewilding introductions.
Current Scientific Basis for Trophic Rewilding
Trophic Cascades.
Trophic cascades offer a key theoretical framework for trophic rewilding. An increasing body of literature documents the existence of trophic cascades, where apex consumers shape ecosystems via effects on prey and other resources, as well as competitors, and their multidirectional propagation through food webs (6, 27). These apex consumers are often large-bodied carnivores and herbivores. Trophic cascades have been truncated by humans, with strong effects on ecosystems and often negative consequences for biodiversity (6, 7). Trophic rewilding attempts to remedy this by restoring the missing top-down–mediated processes. We note that these need not all be strictly trophic, but also include associated processes (e.g., nonfeeding related disturbances, such as wallows) (28).
Since the expansion of Homo sapiens across the world, megafaunas have undergone progressive simplification (2, 4), a process that is still ongoing (5). This loss has had two, sometimes overlapping phases: (i) severe late Pleistocene and early Holocene losses of a broad range of megafauna, often leading to complete loss of herbivores ≥1,000 kg (2, 4); (ii) continuing range contractions in remaining continental megafauna from the mid-Holocene onwards (29–31), including further rare global extinctions (32). On islands, the first phase often occurred in the mid- or late Holocene (3). These losses have had strong ecosystem effects, notably via trophic cascades. The initial extinctions affected vegetation, fire regimes, biogeochemical cycling, and possibly even climate (33–36), and are linked to losses among dependent species, such as scavenging birds and dung beetles (37–40). Later and current megafauna losses have also had strong ecological impacts via, for example, abundance increases in medium-sized herbivores (41), mesopredator release (7), and disperser losses (42). Although defaunation proceeds in most places (5), some regions are recovering: for example, Europe (43, 44).
Large carnivores and herbivores play important roles in trophic cascades. Although now rare because of persecution, large carnivores were ubiquitous until recently (6, 7). Large carnivores may control the density and behavior of herbivores and mesopredators (7). For example, wolves limit densities of nonmigratory deer and may do so even more strongly when co-occurring with other large predators (45). Herbivores weighing ≥1,000 kg (megaherbivores) are thought to be largely immune to adult predation and limited by resources (46, 47). They can have strong vegetation and ecosystem impacts because of their abundance and sheer size (28, 46). Formerly cosmopolitan, they are now limited to parts of Africa and Asia (Fig. 1). It is possible that predation on their juveniles may have provided greater regulation of their abundances during the Pleistocene (48), although stable isotope evidence suggests that megaherbivores rarely formed a major part of Pleistocene carnivore diets (49, 50). Intermediate-size (100–999 kg) herbivores are also not always top-down–regulated. For example, in some African savanna ecosystems large herbivores >150 kg experience only limited predation and are largely bottom-up regulated (47). More generally, herd-forming migratory ungulates often escape predation regulation (47, 51). Irrespective of top-down or bottom-up regulation, large herbivores can have pervasive ecosystem effects, impacting primary production, nutrient cycling, disturbance regimes, habitat heterogeneity, and seed dispersal (28, 42, 52, 53).
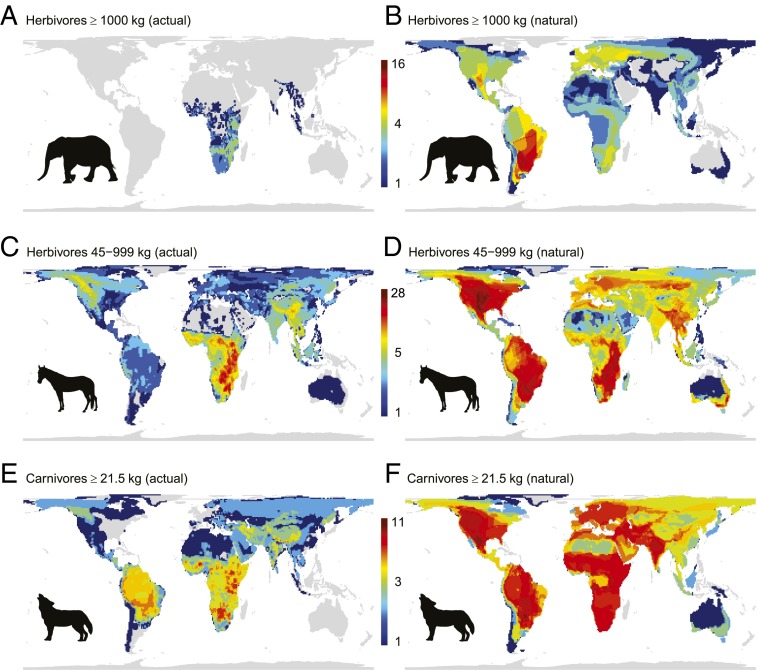
Fig. 1.
Current and estimated present-natural diversity patterns for (A and B) megaherbivores (≥1,000 kg), (C and D) large herbivores (45–999 kg), and (E and F) large carnivores (>21.5 kg). The term “present-natural” refers to the state that a phenomenon would be in today in the complete absence of human influence through time (111). For this mapping, omnivores were classified as carnivores when meat constitutes a major part of their diet and as herbivores otherwise.
Pre-extinction megafaunas in large parts of the world were as species-rich as any extant megafauna, with multiple species of megaherbivores and high diversities of intermediate-size herbivores and large carnivores (Fig. 1). The exact functioning of trophic cascades in these ecosystems is uncertain (54), although intact current African ecosystems are useful models (46, 47). Importantly, trophic complexity (55), such as diversity within trophic levels, can be important (7, 56). It is clear, however, that large herbivores were numerically abundant in the Late Pleistocene and had strong impacts on vegetation structure and ecosystem dynamics (35, 57, 58).
Lessons From Major Trophic Rewilding Experiments.
Explicit trophic rewilding experiments are few in number, but have provided important lessons. The reintroduction of wolves to Yellowstone National Park in the mid-1990s is perhaps the most widely known example (59). The wolves restored a tritrophic cascade, where direct predation and behavioral impacts on American elk (Cervus elaphus) have increased regeneration of Populus and Salix spp., with indirect effects on other wildlife and geomorphology (60). Controversy exists over the exact role of wolves in these dynamics (59), but similar effects are reported elsewhere in North America (61, 62) and Europe (63). Other experiments have focused on large herbivores. The 56-km2 Oostvaardersplassen experiment in the Netherlands was initiated at a time when closed woodland was assumed to be the naturally dominant vegetation in much of Europe. In 1983, feral populations of primitive cattle and horse breeds were introduced as proxies for their extirpated wild ancestors, along with red deer (Cervus elaphus), with a major—successfully achieved—aim being maintaining grasslands in drier parts as feeding habitats for greylag geese (Anser anser) (64). The geese are themselves important for local bird diversity, because during molting they withdraw to the marshy parts, grazing reed beds and creating a mosaic of shallow water and vegetation that facilitates many other species (64). Largely regulated by food availability, the herbivores have strongly reduced the predominantly nonthorny woody vegetation (64–66). In time a dynamic tree–grassland mosaic might establish if grazing refuges develop (65) [e.g., via temporary declines in herbivore abundance and thorn scrub establishment (66)]. In Siberia, bison and other herbivores have been reintroduced to a site, with the goal of restoring grazing-dependent mammoth-steppe vegetation (67). Results to date indicate a shift from shrub- to grass-dominance in experimental enclosures (68). In recent years, many more trophic rewilding projects are being implemented (Fig. 2). Notably, introduction of nonnative large tortoises as replacements for extinct species is being tested on several oceanic islands (25). These constitute functional megaherbivore reintroductions, as tortoises were the largest native vertebrates on many islands (69). Several studies have documented their successful establishment (70) and found them to improve dispersal and recruitment in endemic trees (71) and suppress invasive plants (72).
Fig. 2.
Examples of reintroductions or extant functional counterparts to replace extinct species in ongoing or proposed rewilding projects on islands and continents: Island examples include (Bottom to Top): Snares Island snipe (Coenocorypha huegeli) replacing the South Island snipe (Coenocorypha iredalei) on Putauhinu Island, New Zealand; giant Aldabra tortoise (Aldabrachelys gigantea) replacing giant Cylindraspis tortoises on Rodrigues Island, Mauritius; African sulcata tortoises (Centrochelys sulcata) replacing a flightless bird, the moa-nalo (Chelychelynechen quassus) on Kaua’i, Hawai’i. Continental examples include (Bottom to Top): Wild boar (Sus scrofa), experimental reintroduction to a fenced rewilding site in the Scottish Highlands; European Bison (Bison bonasus), an increasing number of rewilding reintroductions of this species are being implemented across Europe, here in Vorup Enge, Denmark (all ongoing projects); Asian elephant (Elephas maximus) replacing straight-tusked elephant (Elephas antiquus) in Eskebjerg Vesterlyng, Denmark (3-d pilot experiment, 2008). Approximate times because local or regional extinction of the original taxa are given. Images courtesy of (Top Right) J. Kunstmann and (Bottom Left) C. Miskelly.
State of Literature Focused on Trophic Rewilding.
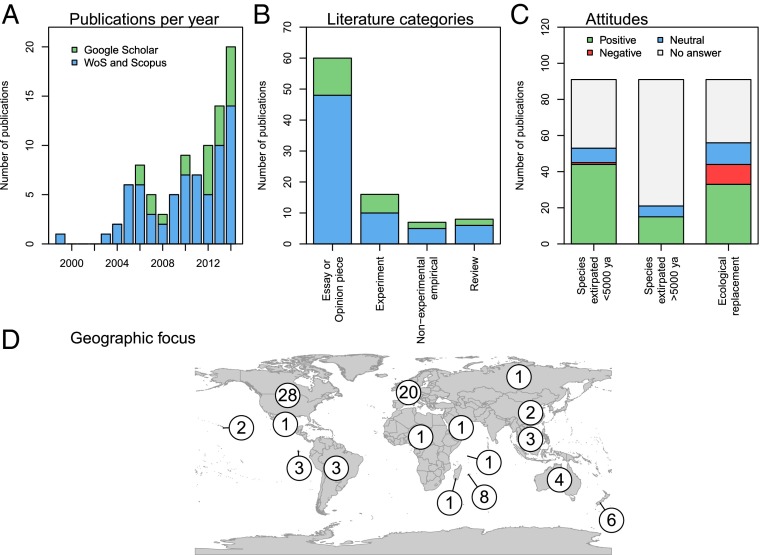
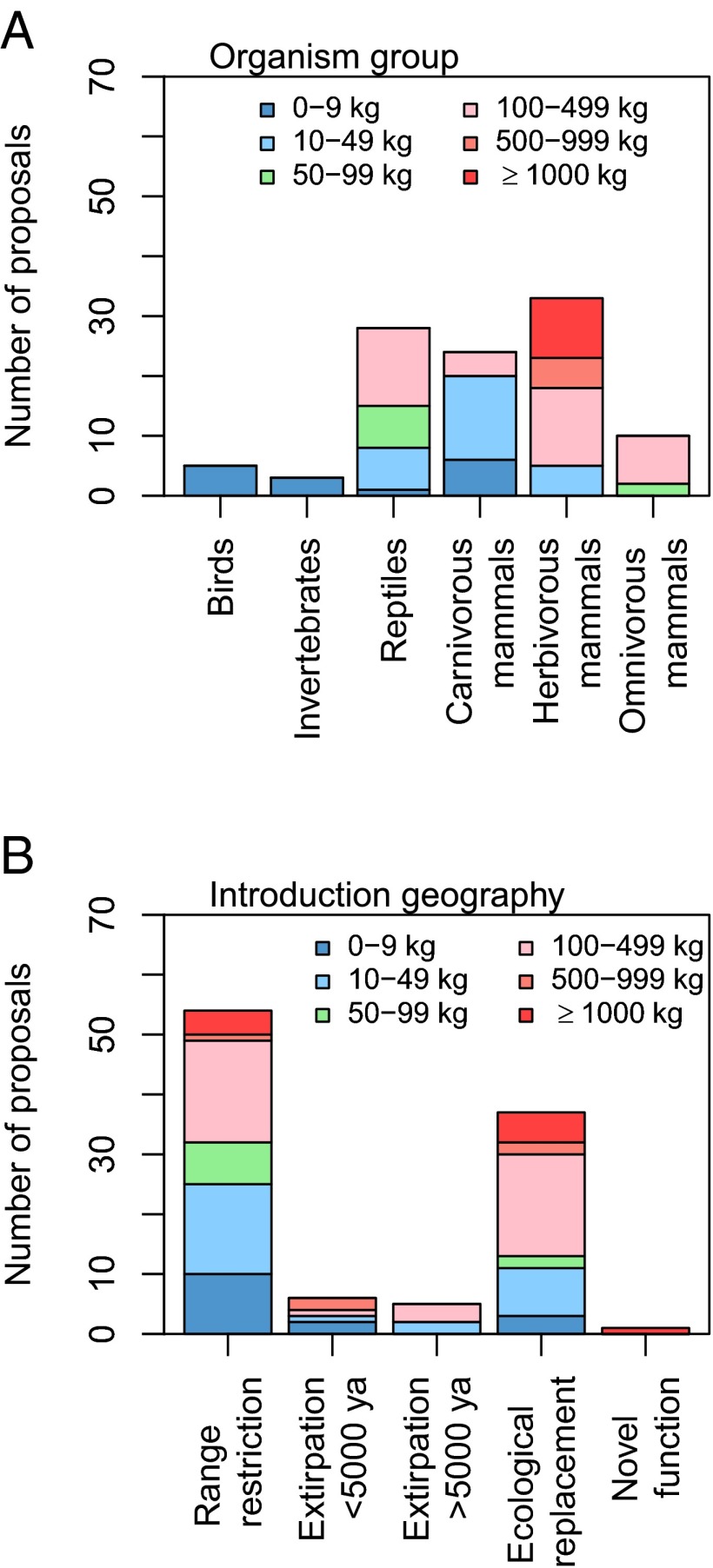
To further assess the state of trophic rewilding science, we carried out a systematic review of the international scientific literature, identifying 91 rewilding-focused publications (Methods). Despite an ongoing increase in publications (Fig. 3A), empirical studies are few whereas essays and opinion pieces predominate (Fig. 3B and SI Appendix, Table S1). There is strong geographic bias in the literature and the featured projects, with most focusing on North America, Europe, and oceanic islands (Fig. 3D and SI Appendix, Tables S1 and S2). A majority were positive to reintroduction of species extirpated regionally within the last 5,000 y (Fig. 3C). Support for reintroducing species extirpated >5,000 y ago or introducing ecological replacements for extinct species was weaker (Fig. 3C). Large carnivorous and herbivorous mammals, as well as reptiles (tortoises), are the focus of the current literature (Fig. 4A and SI Appendix, Table S3), reflecting the importance attributed to them in generating trophic cascades and, for tortoises, also ease of implementation (25). Furthermore, most cases concern reintroduction of species regionally or locally extirpated during the last 5,000 y (Fig. 4B), likely reflecting their greater acceptance. Still, a substantial proportion concerns introduction of ecological replacements (Fig. 4B), mostly substituting species extinct <5,000 y ago (SI Appendix, Table S3), illustrating attention to rewilding with large tortoises on islands.
Fig. 3.
Characteristics of international scientific publications focused on trophic rewilding (n = 91) (see Methods for further details on the systematic review): (A) number published per year, (B) literature categories, (C) attitude toward rewilding using reintroduction of species extirpated <5,000 y, reintroduction of species extirpated >5,000 y ago, or ecological replacements, (D) geographical focus.
Fig. 4.
Characteristics of species mentioned for rewilding introductions in international scientific publications focused on trophic rewilding (n = 91) (see Methods for further details on the systematic review). (A) Organism group and body mass. (B) Introduction geography [range-restricted species (species extirpated locally, but still extant within the focal zoogeographic region, with reduced range), species extirpated <5,000 y ago (species completely extirpated from the focal region within the last 5,000 y, but still extant elsewhere), species extinct >5,000 y ago (species completely extirpated from the focal region more than 5,000 y ago, but still extant elsewhere), ecological replacement for a globally extinct species, novel function (introduction to achieve novel ecological function without a past analog)], and body mass.
Among the empirical studies, many cover the most studied island rewilding experiments to date, the introduction of exotic tortoises (Astrochelys radiata, Aldabrachelys gigantea) on small islands near Mauritius as replacements for extinct giant tortoises (Cylindrapsis) (e.g., refs. 25, 70–72), and giant tortoise (Chelonoidis spp.) translocations within the Galapagos Islands (e.g., ref. 73). These studies document that large and giant tortoises are low-risk, high-impact rewilding candidates that provide key ecological functions as dispersers of large seeds, herbivores, and disturbance agents. These factors and the initial successes have led to proposals for tortoise rewilding efforts on other islands (25). An important point comes from a >30-y reintroduction project for a Galapagos tortoise (Chelonoidis hoodensis) to Española Island, showing successful population establishment but limited ecosystem recovery because of self-reinforcing past anthropogenic vegetation changes (74). The authors conclude that functional reinstatement of ecosystem engineers may necessitate large-scale habitat restoration efforts jointly with population restoration (74). The nontortoise empirical studies cover a broad range of topics, such as climatic suitability, biodiversity impacts, and public acceptance, but are too few and scattered in focus to allow generalization (SI Appendix, Table S1).
Insights from Unintentional Trophic Rewilding.
Building on Wilder et al. (75), we define unintentional trophic rewilding as introduction of a species or functional type of relevancy for restoring trophic cascades done without knowledge that it was once native. Large-scale examples include the reintroduction of equids (Equus spp.) to the New World (75, 76), fallow deer (Dama dama) across Europe (76), muskox (Ovibos moschatus) across the Eurasian and North American arctic (76, 77), and certain introduced birds in New Zealand (78). Unintentional rewilding offers an underexploited research opportunity, often on larger spatiotemporal scales than possible with experiments. North American feral horses constitute one of the best-studied cases. Local effects on vegetation and wildlife have been documented (79, 80), but our current understanding on how feral horses influence ecosystems nevertheless remains limited [e.g., in terms of geographic variability, scale dependency, and predation effects (81)]. Studies of introduced species generally also have potential to inform rewilding; for example, as seen in a recent study comparing native and introduced plant mutualists, concluding that ecological replacements may benefit native plants when native mutualists have been lost (82). Exemplifying this, feral pigs (Sus scrofa) in the Pantanal have helped restore dispersal of plants adapted for dispersal by extinct megafauna (83, 84).
Insights from Wildlife Comebacks.
Another underexploited source of information comes from spontaneous wildlife comebacks. After millennia of declines (2, 3), large mammals are making a comeback in Europe and North America, with legal protection, targeted species conservation, supportive public opinion, and land abandonment as drivers (e.g., 43, 44). It is important to assess the impacts. For example, are the recoveries of apex predators sufficient to restore trophic cascades in human-dominated landscapes? Outside large reserves, human impacts could limit their ecological role (85). However, there are examples of restored trophic cascades in such landscapes (43). The recovery of large herbivore populations has been even more pronounced than carnivores and often has strong ecological effects. Deer have expanded dramatically in many areas and can suppress tree regeneration as well as affect plant and animal diversity (41, 86), and not always negatively (41, 87). Again, human activities are likely to influence these effects.
Priorities for Research on Trophic Rewilding
There is a clear need for developing a larger, more systematic research program to develop the scientific basis for trophic rewilding, with trophic cascades as a key framework (6, 27). A difficulty is that to assess its role as a large-scale restoration tool, large experimental areas are needed (cf. ref. 88). If ecological research received a level of funding comparable to that of space research, it would be easy to conduct strong factorial-design experiments in replicated 100- to 1,000-km2 enclosures to rigorously test key issues. Pragmatically, an increasing number of rewilding programs are being implemented (Fig. 2), providing important opportunities if designed to allow scientific assessment of their effects and with a monitoring program to follow their dynamics (e.g., ref. 89). It is important that assessments provide broad coverage of biodiversity and ecosystem processes: for example, also more rarely assessed species-rich groups, such as insects and fungi. In the following, we discuss key priorities for rewilding research.
Global Scope.
The potential ecological impact of trophic rewilding should increase with the degree to which megafaunas have been impoverished. We provide a global estimate of such deficits (Fig. 1) by comparing the current species richness for three key terrestrial megafauna functional groups with their estimated species richness given the present climate, but in the absence of Late Pleistocene and Holocene extinctions and extirpations: megaherbivores (≥1,000 kg body mass), large herbivores (45–999 kg), and large carnivores (≥21.5 kg), corresponding to the size where predators switch from small to large prey (90). Megaherbivores are absent from most areas today, except in increasingly small parts of Southeast Asia and Africa. However, most continental areas had megaherbivore richness comparable to or higher than those currently seen in Africa and Asia (Fig. 1). Because proboscideans were members of this guild in all regions except Australia, extant elephant species are relevant to consider as ecological replacements in most areas (16, 18, 91). Major diversity deficits are also widespread for large carnivores and large herbivores, although most areas still have some representatives (Fig. 1). The strongly reduced diversities in all three groups in most regions suggest a global relevancy of trophic rewilding, and a need for overcoming the strong geographic research biases.
Trophic Complexity.
The complexity of restored trophic networks is likely highly important (55), but not well understood in the context of rewilding: that is, how do simple pair-wise systems, such as a wolf-red deer, differ from complex networks involving multiple carnivores and a broad range of herbivores, some partially immune to predation (6, 45, 47)? Importantly, outside Africa and Asia no experiment has yet assessed the effects of restoring full Pleistocene-like trophic complexity. Notably, experimental rewilding studies on megaherbivores are lacking, despite their high functional importance and former omnipresence.
Landscape Setting and Interplay with Society.
Trophic rewilding projects range from small fenced biotopes to large landscapes and are situated in a variety of environments and landscape structures. As landscape factors—such as area, climate, environmental gradients, and disturbance, as well as societal circumstances—may be highly important for the ecology of megafauna-rich ecosystems (e.g., refs. 47 and 92), research needs to address how this restoration approach can best be implemented in different landscape settings. One important issue is how constraints on animal movement (e.g., seasonal migration), such as fences, will affect its ecological effects, and how these constraints can be overcome if needed (93). Although fences sometimes have negative effects [e.g., limiting the ability of large herbivores to disperse seeds across landscapes (cf. ref. 94)], they may also have positive effects, by excluding negative anthropogenic effects (93). More generally, it needs to be addressed how trophic rewilding integrated with other conservation approaches (92) may be best implemented to promote biodiverse ecosystems that are as self-regulating as possible within the constraints of limited space and human needs in urban or agricultural areas. Whether a land-sparing or land-sharing approach should be adopted to achieve these goals remains an open question (95, 96), although some land sparing seems essential (96). Related to this, there is a need to assess how rewilding will impact ecosystem services. Agricultural land is a dominant land use over much of Earth’s terrestrial surface (97) and returning it to wild land will likely decrease the provision of food but restore other services, particularly regulating and cultural services (98). Understanding the balance of such costs and benefits and the factors determining them will be important for guiding policies on rewilding. Finally, in landscapes dominated by nonanalog novel ecosystems (99), it may also be relevant to consider rewilding introductions to establish novel trophic cascades to promote self-regulating biodiverse ecosystems (100). Such a radical approach would clearly require a solid scientific base.
Management Targets and Tools.
Many trophic rewilding projects most logically should be implemented as open-ended conservation projects, acknowledging the limits of our knowledge and unavoidable future changes, for example, in climate (89). Nevertheless, direct management decisions will continue to play an important role in many cases because of societal requirements or spatial constraints. Hence, research on functional rewilding targets is needed (e.g., ref. 35), preferably combining paleoecological and historical evidence with experimental studies. Although trophic rewilding may benefit natural ecosystems and recreational values, it may also cause human–wildlife conflicts: for example, damages to crops and livestock. There is a need to collect empirical evidence for effectiveness and consequences of tools—such as culling, targeted feeding, and fencing—to maximize benefits and reduce costs (93, 101, 102).
Climate Change.
Strong changes in climate are expected for the next 100 y and beyond, and are already now driving shifts in species ranges and ecosystem changes (103). These dynamics will impact all conservation management, including trophic rewilding. It will be important to assess how climate changes may affect the outcome of rewilding efforts, for example altering the suitability of a given locality for a candidate species. Trophic rewilding has the potential to help mitigate negative effects of climate change. Free-ranging large herbivores will increase seed-dispersal distances for many plants (94), increasing their ability to track climate. Trophic cascades established by rewilding may also sometimes halt detrimental ecosystem changes. Reindeer (Rangifer tarandus) and muskoxen (Ovibos moschatus) can limit shrub expansion in tundras, reducing negative effects on herbaceous plants (104). It is even hypothesized that restoration of Pleistocene-like megafaunas in the Arctic may re-establish grasslands, slow permafrost thawing, and increase albedo, reducing warming (68). However, it is also plausible that megafauna restoration in some cases may trade-off against climate change mitigation, decreasing carbon sequestration (105) and increasing methane emissions (33). There is a strong need for research to further our understanding of these issues.
Species Selection for Trophic Rewilding
Systematic Framework.
Introducing a species into an ecosystem to restore a process inherently involves uncertainty. Therefore, a science-based decision framework that allows practitioners to systematically evaluate potential costs and benefits of a given rewilding introduction would be of high utility. Existing guidelines for conservation translocations are an appropriate starting point, providing best practices on aspects, such as monitoring, feasibility assessment, and release strategies (106). However, guidelines for the key aspect of species selection for rewilding are lacking. A systematic framework should consider three aspects: (i) Match between the ecology of the candidate species and the focal functions to be restored, with functional traits as an important source of information. (ii) Phylogenetic relatedness, to restore the evolutionary potential of a lineage but also to capture subtle functional aspects. Still, there may be relevant ecological replacements even when no closely related species is available (100, 107). (iii) Suitability of the present and forecasted future climate, ecosystem, and societal circumstances to accommodate the candidate species (108). This would include evaluating conservation and societal benefits and risks.
Integrating Synthetic Biology and Rewilding.
Although trophic rewilding has focused on reintroducing regionally extirpated species or ecological replacements for extinct species, an alternative approach that leverages synthetic biology (109, 110) is emerging. It appears likely that it will become possible to engineer organisms to resemble extinct species genetically, phenotypically, or functionally (108, 110). It therefore makes sense to begin integrating the discipline with trophic rewilding (110). Synthetic biology could become a powerful component of trophic rewilding by overcoming limits to what can be achieved with extant species, as a substantial proportion of extinct species have no close substitutes: for example, megaherbivores capable of tolerating boreal and arctic climates. Hence, a framework for integrating synthetic biology and trophic rewilding science is needed to evaluate risks and benefits (108).
Outlook
We believe trophic rewilding has strong scope for ecological restoration in the Anthropocene, to remedy defaunation and restore trophic cascades that promote self-regulating biodiverse ecosystems. Notably, it is clear that it can have strong ecological effects and has worldwide relevancy (6). Dense human populations provide an obvious challenge, but successful trophic rewilding projects and spontaneous large-animal comebacks in densely populated regions (26, 43, 44) illustrate its potential also as reconciliatory approach (95). There is a strong need to develop a broad empirical research agenda, as empirical studies are rare. Ideally such research should include large-scale replicated experiments to allow rigorous hypothesis testing. However, applied rewilding projects should also whenever possible be designed with hypothesis testing in mind and include science-based monitoring to allow assessment of their effects. Trophic cascades provide a key theoretical framework for trophic rewilding, but a better, predictive understanding of how they function in megafauna-rich ecosystems with high trophic complexity is needed. Other key issues concern their interplay with landscape settings, societal priorities, human activities, as well as climate change. Finally, we see much potential in integrating trophic rewilding with related approaches, notably passive rewilding (spontaneous ecological dynamics without management) (12, 14, 15), abiotic rewilding (restoration of natural physical processes, e.g., river dynamics), and open-ended management (89).
Methods
We compared current mammal distributions following the International Union for Conservation of Nature with estimated present-natural distributions. The term “present-natural” refers to the state that a phenomenon would be in today in the complete absence of human influence through time (111). Present-natural distributions were estimated for all mammal species with occurrence records from within the last 130,000 y [following a recently revised taxonomy (112)], as the far majority of extinctions in this period can be linked to Homo sapiens’ global expansion (2–4). A full description of the estimated ranges can be found in Faurby and Svenning (113). For extant species, these were based on historical range estimates when available or alternatively on a combination of historic knowledge and climate. For prehistoric extinct species, ranges were generally based on a co-occurrence approach, assuming that they would have responded to the late-Quaternary climatic changes similarly to the species with which they used to co-occur. Hence, we estimated their present-natural distributions based on those of the extant species with which they co-occurred. To evaluate this approach, we compared the range of 39 extant species in North America estimated with this approach to their historical (pre-Columbus) distributions. The correlation between present-natural and historic diversity (ρ = 0.856) was higher than between historic and current diversity (ρ = 0.762) (113). The resulting megafauna diversity patterns are broadly concordant with earlier studies (114).
The systematic review was based on English-language scientific papers published by December 2014, found in Web of Science and Scopus using the search terms “rewilding” and “re-wilding.” Additionally, to expand coverage, for the 10 highest-cited papers, we used Google Scholar to provide lists of citing articles and reviewed these, too. To ensure that the systematic review was focused on publications on rewilding in the sense of trophic rewilding as defined here, we only included articles treating conservation translocations explicitly aimed to restore ecological function. For each paper we then assessed: (i) author attitude towards rewilding, separately for the following introduction geographies: reintroductions of extant species that have been completely extirpated from the focal zoogeographical region within the last 5,000 y, reintroductions of extant species completely extirpated from the focal region >5,000 y ago, or introductions of ecological replacements for globally extinct species; (ii) type of publication (essay or opinion piece; review; experiment; nonexperimental empirical); (iii) geographic focus; (iv) rewilding projects mentioned; and (v) species for rewilding mentioned. Species were characterized by organism group, diet, introduction geography (as above, plus reintroductions of locally extirpated species still extant elsewhere within a given region, and introductions to achieve novel ecological functions), body mass, and time since disappearance from the focal zoogeographical region. The SI Appendix provides an overview of the methodology for the systematic review as well as the resulting data.
Supplementary Material
Acknowledgments
This work was supported in part by European Research Council Grant ERC-2012-StG-310886-HISTFUNC (to J.-C.S.); Aarhus University and the Aage V. Jensen Foundations (P.B.M.P.); and Conselho Nacional de Desenvolvimento Científico and Fundação de Amparo à Pesquisa do Estado de São Paulo (M.G.). We consider this article a contribution to the Danish National Research Foundation Niels Bohr professorship project Aarhus University Research on the Anthropocene.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. Y.M. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1502556112/-/DCSupplemental.
References
- 1.Crutzen PJ. Geology of mankind. Nature. 2002;415(6867):23. doi: 10.1038/415023a. [DOI] [PubMed] [Google Scholar]
- 2.Sandom C, Faurby S, Sandel B, Svenning J-C. Global late Quaternary megafauna extinctions linked to humans, not climate change. Proc Biol Sci. 2014;281(1787):20133254. doi: 10.1098/rspb.2013.3254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Turvey ST, Fritz SA. The ghosts of mammals past: Biological and geographical patterns of global mammalian extinction across the Holocene. Philos Trans R Soc Lond B Biol Sci. 2011;366(1577):2564–2576. doi: 10.1098/rstb.2011.0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barnosky AD, Koch PL, Feranec RS, Wing SL, Shabel AB. Assessing the causes of late Pleistocene extinctions on the continents. Science. 2004;306(5693):70–75. doi: 10.1126/science.1101476. [DOI] [PubMed] [Google Scholar]
- 5.Dirzo R, et al. Defaunation in the Anthropocene. Science. 2014;345(6195):401–406. doi: 10.1126/science.1251817. [DOI] [PubMed] [Google Scholar]
- 6.Estes JA, et al. Trophic downgrading of planet Earth. Science. 2011;333(6040):301–306. doi: 10.1126/science.1205106. [DOI] [PubMed] [Google Scholar]
- 7.Ripple WJ, et al. Status and ecological effects of the World’s largest carnivores. Science. 2014;343(6167):1241484. doi: 10.1126/science.1241484. [DOI] [PubMed] [Google Scholar]
- 8.Terborgh J, Estes JA, editors. Trophic Cascades: Predators, Prey, and the Changing Dynamics of Nature. Island Press; Washington, DC: 2010. [Google Scholar]
- 9.Soule M, Noss R. Rewilding and biodiversity: Complementary goals for continental conservation. Wild Earth. 1998;8(3):1–11. [Google Scholar]
- 10.Zimov SA, et al. Steppe-tundra transition: A herbivore-driven biome shift at the end of the Pleistocene. Am Nat. 1995;146(5):765–794. [Google Scholar]
- 11.Baerselman F, Vera F. Nature Development. An Exploratory Study for the Construction of Ecological Networks. Ministry of Agriculture, Nature Management and Fisheries; The Hague, The Netherlands: 1995. [Google Scholar]
- 12.Jørgensen D. Rethinking rewilding. Geoforum. 2015 doi: 10.1016/j.geoforum.2014.11.016. [DOI] [Google Scholar]
- 13.Seddon PJ, Griffiths CJ, Soorae PS, Armstrong DP. Reversing defaunation: Restoring species in a changing world. Science. 2014;345(6195):406–412. doi: 10.1126/science.1251818. [DOI] [PubMed] [Google Scholar]
- 14.Navarro LM, Pereira HM. Rewilding abandoned landscapes in Europe. Ecosystems (N Y) 2012;15(6):900–912. [Google Scholar]
- 15.Schnitzler A. Towards a new European wilderness: Embracing unmanaged forest growth and the decolonisation of nature. Landscape Urban Plan. 2014;126:74–80. [Google Scholar]
- 16.Donlan CJ, et al. Pleistocene rewilding: An optimistic agenda for twenty-first century conservation. Am Nat. 2006;168(5):660–681. doi: 10.1086/508027. [DOI] [PubMed] [Google Scholar]
- 17.Donlan J, et al. Re-wilding North America. Nature. 2005;436(7053):913–914. doi: 10.1038/436913a. [DOI] [PubMed] [Google Scholar]
- 18.Galetti M. Parks of the Pleistocene: Recreating the Cerrado and the Pantanal with megafauna. Nat Conserv. 2004;2(1):93–100. [Google Scholar]
- 19.Smith FA, et al. The evolution of maximum body size of terrestrial mammals. Science. 2010;330(6008):1216–1219. doi: 10.1126/science.1194830. [DOI] [PubMed] [Google Scholar]
- 20.Nenzén HK, Montoya D, Varela S. The impact of 850,000 years of climate changes on the structure and dynamics of mammal food webs. PLoS One. 2014;9(9):e106651. doi: 10.1371/journal.pone.0106651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carrasco MA, Barnosky AD, Graham RW. Quantifying the extent of North American mammal extinction relative to the pre-anthropogenic baseline. PLoS One. 2009;4(12):e8331. doi: 10.1371/journal.pone.0008331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Janzen DH, Martin PS. Neotropical anachronisms: The fruits the gomphotheres ate. Science. 1982;215(4528):19–27. doi: 10.1126/science.215.4528.19. [DOI] [PubMed] [Google Scholar]
- 23.Hayward MW. Conservation management for the past, present and future. Biodivers Conserv. 2009;18(4):765–775. [Google Scholar]
- 24.Sandom C, Donlan CJ, Svenning J-C, Hansen D. Rewilding. In: Macdonald DW, Willis KJ, editors. Key Topics in Conservation Biology 2. Wiley-Blackwell; Chichester, UK: 2013. pp. 430–451. [Google Scholar]
- 25.Hansen DM, Donlan CJ, Griffiths CJ, Campbell KJ. Ecological history and latent conservation potential: Large and giant tortoises as a model for taxon substitutions. Ecography. 2010;33(2):272–284. [Google Scholar]
- 26.Reardon S. Rewilding: The next big thing? New Sci. 2014;221(2958):40–43. [Google Scholar]
- 27.Terborgh J, et al. The role of top carnivores in regulating terrestrial ecosystems. In: Soulé ME, Terborgh J, editors. Continental Conservation—Scientic Foundations of Regional Reserve Networks. Island Press; Washington, DC: 1999. pp. 39–64. [Google Scholar]
- 28.Haynes G. Elephants (and extinct relatives) as earth-movers and ecosystem engineers. Geomorphology. 2012;157–158:99–107. [Google Scholar]
- 29.Morrison JC, Sechrest W, Dinerstein E, Wilcove DS, Lamoreux JF. Persistence of large mammal faunas as indicators of global human impacts. J Mammal. 2007;88(6):1363–1380. [Google Scholar]
- 30.Schmölcke U, Zachos FE. Holocene distribution and extinction of the moose (Alces alces, Cervidae) in Central Europe. Mamm Biol. 2005;70(6):329–344. [Google Scholar]
- 31.Laliberte AS, Ripple WJ. Range contractions of North American carnivores and ungulates. Bioscience. 2004;54(2):123–138. [Google Scholar]
- 32.Crees JJ, Turvey ST. Holocene extinction dynamics of Equus hydruntinus, a late-surviving European megafaunal mammal. Quat Sci Rev. 2014;91:16–29. [Google Scholar]
- 33.Smith FA, Elliott SM, Lyons SK. Methane emissions from extinct megafauna. Nat Geosci. 2010;3(6):374–375. [Google Scholar]
- 34.Gill JL. Ecological impacts of the late Quaternary megaherbivore extinctions. New Phytol. 2014;201(4):1163–1169. doi: 10.1111/nph.12576. [DOI] [PubMed] [Google Scholar]
- 35.Sandom CJ, Ejrnæs R, Hansen MDD, Svenning J-C. High herbivore density associated with vegetation diversity in interglacial ecosystems. Proc Natl Acad Sci USA. 2014;111(11):4162–4167. doi: 10.1073/pnas.1311014111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Doughty CE, Wolf A, Field CB. Biophysical feedbacks between the Pleistocene megafauna extinction and climate: The first human-induced global warming? Geophys Res Lett. 2010;37(15):L15703. [Google Scholar]
- 37.Cenizo MM, Agnolin FL, Pomi LH. A new Pleistocene bird assemblage from the southern Pampas (Buenos Aires, Argentina) Palaeogeogr Palaeoclimatol Palaeoecol. 2015;420:65–81. [Google Scholar]
- 38.Tyrberg T. The Late Pleistocene continental avian extinction—An evaluation of the fossil evidence. Oryctos. 2008;7:249–269. [Google Scholar]
- 39.Sánchez MV, Genise JF, Bellosi ES, Román-Carrión JL, Cantil LF. Dung beetle brood balls from Pleistocene highland palaeosols of Andean Ecuador: A reassessment of Sauer’s Coprinisphaera and their palaeoenvironments. Palaeogeogr Palaeoclimatol Palaeoecol. 2013;386:257–274. [Google Scholar]
- 40.Chamberlain CP, et al. Pleistocene to recent dietary shifts in California condors. Proc Natl Acad Sci USA. 2005;102(46):16707–16711. doi: 10.1073/pnas.0508529102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Côté SD, Rooney TP, Tremblay J-P, Dussault C, Waller DM. Ecological impacts of deer overabundance. Annu Rev Ecol Evol Syst. 2004;35:113–147. [Google Scholar]
- 42.Campos-Arceiz A, Blake S. Megagardeners of the forests—The role of elephants in seed dispersal. Acta Oecol. 2011;37(6):542–553. [Google Scholar]
- 43.Chapron G, et al. Recovery of large carnivores in Europe’s modern human-dominated landscapes. Science. 2014;346(6216):1517–1519. doi: 10.1126/science.1257553. [DOI] [PubMed] [Google Scholar]
- 44.Deinet S, et al. Wildlife Comeback in Europe: The Recovery of Selected Mammal and Bird Species. Final Report to Rewilding Europe by ZSL, Birdlife International and the European Bird Census Council. Zoological Society of London; London: 2013. [Google Scholar]
- 45.Ripple WJ, Beschta RL. Large predators limit herbivore densities in northern forest ecosystems. Eur J Wildl Res. 2012;58(4):733–742. [Google Scholar]
- 46.Owen-Smith N. Pleistocene extinctions: The pivotal role of megaherbivores. Palaeobiology. 1987;13(3):351–362. [Google Scholar]
- 47.Hopcraft JG, Olff H, Sinclair AR. Herbivores, resources and risks: Alternating regulation along primary environmental gradients in savannas. Trends Ecol Evol. 2010;25(2):119–128. doi: 10.1016/j.tree.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 48.Van Valkenburgh B, Hayward MW, Ripple WJ, Meloro C, Roth VL. The impact of large terrestrial carnivores on Pleistocene ecosystems. Proc Natl Acad Sci USA. 2016;113:862–867. doi: 10.1073/pnas.1502554112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bocherens H. Isotopic tracking of large carnivore palaeoecology in the mammoth steppe. Quat Sci Rev. 2015;117:42–71. [Google Scholar]
- 50.Coltrain JB, et al. Rancho La Brea stable isotope biogeochemistry and its implications for the palaeoecology of late Pleistocene, coastal southern California. Palaeogeogr Palaeoclimatol Palaeoecol. 2004;205(3-4):199–219. [Google Scholar]
- 51.Skogland T. What are the effects of predators on large ungulate populations? Oikos. 1991;61(3):401–411. [Google Scholar]
- 52.Doughty CE, Wolf A, Malhi Y. The legacy of the Pleistocene megafauna extinctions on nutrient availability in Amazonia. Nat Geosci. 2013;6(9):761–764. [Google Scholar]
- 53.Hobbs NT. Modification of ecosystems by ungulates. J Wildl Manage. 1996;60(4):695–713. [Google Scholar]
- 54.Ripple WJ, Van Valkenburgh B. Linking top-down forces to the Pleistocene megafaunal extinctions. Bioscience. 2010;60(7):516–526. [Google Scholar]
- 55.Duffy JE, et al. The functional role of biodiversity in ecosystems: Incorporating trophic complexity. Ecol Lett. 2007;10(6):522–538. doi: 10.1111/j.1461-0248.2007.01037.x. [DOI] [PubMed] [Google Scholar]
- 56.Waldram MS, Bond WJ, Stock WD. Ecological engineering by a mega-grazer: White rhino impacts on a South African savanna. Ecosystems (N Y) 2008;11(1):101–112. [Google Scholar]
- 57.Gill JL, Williams JW, Jackson ST, Lininger KB, Robinson GS. Pleistocene megafaunal collapse, novel plant communities, and enhanced fire regimes in North America. Science. 2009;326(5956):1100–1103. doi: 10.1126/science.1179504. [DOI] [PubMed] [Google Scholar]
- 58.Rule S, et al. The aftermath of megafaunal extinction: Ecosystem transformation in Pleistocene Australia. Science. 2012;335(6075):1483–1486. doi: 10.1126/science.1214261. [DOI] [PubMed] [Google Scholar]
- 59.Dobson AP. Yellowstone wolves and the forces that structure natural systems. PLoS Biol. 2014;12(12):e1002025. doi: 10.1371/journal.pbio.1002025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Beschta RL, Ripple WJ. The role of large predators in maintaining riparian plant communities and river morphology. Geomorphology. 2012;157-158:88–98. [Google Scholar]
- 61.Hebblewhite M, Smith DW. Wolf community ecology: Ecosystem effects of recovering wolves in Banff and Yellowstone national parks. In: Musiani M, Boitani L, Paquet P, editors. The World of Wolves: New Perspectives on Ecology, Behavior and Management. Univ of Calgary Press; Calgary, AB, Canada: 2010. pp. 69–120. [Google Scholar]
- 62.Callan R, Nibbelink NP, Rooney TP, Wiedenhoeft JE, Wydeven AP. Recolonizing wolves trigger a trophic cascade in Wisconsin (USA) J Ecol. 2013;101(4):837–845. [Google Scholar]
- 63.Kuijper DPJ, et al. Landscape of fear in Europe: Wolves affect spatial patterns of ungulate browsing in Białowieża Primeval Forest, Poland. Ecography. 2013;36(12):1263–1275. [Google Scholar]
- 64.Vera FWM. Large-scale nature development—The Oostvaardersplassen. British Wildlife. 2009;20(5):28–36. [Google Scholar]
- 65.Smit C, Ruifrok JL, van Klink R, Olff H. Rewilding with large herbivores: The importance of grazing refuges for sapling establishment and wood-pasture formation. Biol Conserv. 2015;182:134–142. [Google Scholar]
- 66.Cornelissen P, Bokdam J, Sykora K, Berendse F. Effects of large herbivores on wood pasture dynamics in a European wetland system. Basic Appl Ecol. 2014;15(5):396–406. [Google Scholar]
- 67.Zimov SA. Pleistocene park: Return of the mammoth’s ecosystem. Science. 2005;308(5723):796–798. doi: 10.1126/science.1113442. [DOI] [PubMed] [Google Scholar]
- 68.Zimov SA, Zimov NS, Tikhonov AN, Chapin FS., III Mammoth steppe: a high-productivity phenomenon. Quat Sci Rev. 2012;57:26–45. [Google Scholar]
- 69.Hansen DM, Galetti M. The forgotten megafauna. Science. 2009;324(5923):42–43. doi: 10.1126/science.1172393. [DOI] [PubMed] [Google Scholar]
- 70.Griffiths CJ, et al. The welfare implications of using exotic tortoises as ecological replacements. PLoS One. 2012;7(6):e39395. doi: 10.1371/journal.pone.0039395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Griffiths CJ, Hansen DM, Jones CG, Zuël N, Harris S. Resurrecting extinct interactions with extant substitutes. Curr Biol. 2011;21(9):762–765. doi: 10.1016/j.cub.2011.03.042. [DOI] [PubMed] [Google Scholar]
- 72.Griffiths CJ, Zuë LN, Jones CG, Ahamud Z, Harris S. Assessing the potential to restore historic grazing ecosystems with tortoise ecological replacements. Conserv Biol. 2013;27(4):690–700. doi: 10.1111/cobi.12087. [DOI] [PubMed] [Google Scholar]
- 73.Hunter EA, Gibbs JP, Cayot LJ, Tapia W. Equivalency of Galápagos giant tortoises used as ecological replacement species to restore ecosystem functions. Conserv Biol. 2013;27(4):701–709. doi: 10.1111/cobi.12038. [DOI] [PubMed] [Google Scholar]
- 74.Gibbs JP, Hunter EA, Shoemaker KT, Tapia WH, Cayot LJ. Demographic outcomes and ecosystem implications of giant tortoise reintroduction to Española Island, Galapagos. PLoS One. 2014;9(10):e110742. doi: 10.1371/journal.pone.0110742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wilder BT, et al. Local extinction and unintentional rewilding of bighorn sheep (Ovis canadensis) on a desert island. PLoS One. 2014;9(3):e91358. doi: 10.1371/journal.pone.0091358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Long JL. Introduced Mammals of the World: Their History, Distribution and Influence. CABI Publishing; Wallingford, UK: 2003. [Google Scholar]
- 77.Litvinov YN. Mammals of the Taymyr Peninsula (biodiversity and organization of communities) Contemp Probl Ecol. 2014;7(6):607–617. [Google Scholar]
- 78.Lee WG, Wood JR, Rogers GM. Legacy of avian-dominated plant-herbivore systems in New Zealand. N Z J Ecol. 2010;34(1):28–47. [Google Scholar]
- 79.Beever E, Tausch R, Thogmartin W. Multi-scale responses of vegetation to removal of horse grazing from Great Basin (USA) mountain ranges. Plant Ecol. 2008;196(2):163–184. [Google Scholar]
- 80.Levin PS, Ellis J, Petrik R, Hay ME. Indirect effects of feral horses on estuarine communities. Conserv Biol. 2002;16(5):1364–1371. [Google Scholar]
- 81.Turner JW, Morrison ML. Influence of predation by mountain lions on numbers and survivorship of a feral horse population. Southwest Nat. 2001;46(2):183–190. [Google Scholar]
- 82.Aslan CE, Zavaleta ES, Croll DON, Tershy B. Effects of native and non-native vertebrate mutualists on plants. Conserv Biol. 2012;26(5):778–789. doi: 10.1111/j.1523-1739.2012.01885.x. [DOI] [PubMed] [Google Scholar]
- 83.Pires MM, et al. Reconstructing past ecological networks: The reconfiguration of seed-dispersal interactions after megafaunal extinction. Oecologia. 2014;175(4):1247–1256. doi: 10.1007/s00442-014-2971-1. [DOI] [PubMed] [Google Scholar]
- 84.Donatti CI, Galetti M, Pizo MA, Guimaraes PR, Jr, Jordano P. Living in the land of ghosts: Fruit traits and the importance of large mammals as seed dispersers in the Pantanal, Brazil. In: Dennis AJ, Green RJ, Schupp EW, Westcott DA, editors. Seed Dispersal: Theory and Its Application in a Changing World. CAB International; Wallingford, UK: 2007. pp. 104–123. [Google Scholar]
- 85.Ordiz A, Bischof R, Swenson JE. Saving large carnivores, but losing the apex predator? Biol Conserv. 2013;168:128–133. [Google Scholar]
- 86.Kuijper DPJ. Lack of natural control mechanisms increases wildlife–forestry conflict in managed temperate European forest systems. Eur J For Res. 2011;130(6):895–909. [Google Scholar]
- 87.Hegland SJ, Lilleeng MS, Moe SR. Old-growth forest floor richness increases with red deer herbivory intensity. For Ecol Manage. 2013;310:267–274. [Google Scholar]
- 88.Newsome TM, et al. Resolving the value of the dingo in ecological restoration. Restor Ecol. 2015;23(3):201–208. [Google Scholar]
- 89.Hughes FMR, et al. Monitoring and evaluating large-scale, ‘open-ended’ habitat creation projects: A journey rather than a destination. J Nat Conserv. 2011;19(4):245–253. [Google Scholar]
- 90.Carbone C, Mace GM, Roberts SC, Macdonald DW. Energetic constraints on the diet of terrestrial carnivores. Nature. 1999;402(6759):286–288. doi: 10.1038/46266. [DOI] [PubMed] [Google Scholar]
- 91.Svenning J-C. ‘Pleistocene re-wilding’ merits serious consideration also outside North America. IBS Newsletter. 2007;5(3):3–9. [Google Scholar]
- 92.Beale CM, et al. Ten lessons for the conservation of African savannah ecosystems. Biol Conserv. 2013;167:224–232. [Google Scholar]
- 93.Somers MJ, Hayward M, editors. Fencing for Conservation: Restriction of Evolutionary Potential or a Riposte to Threatening Processes? Springer Science & Business Media; New York: 2012. [Google Scholar]
- 94.Poschlod P, Bonn S. Changing dispersal processes in the central European landscape since the last ice age: An explanation for the actual decrease of plant species richness in different habitats? Acta Botanica Neerlandica. 1998;47(1):27–44. [Google Scholar]
- 95.Rosenzweig ML. Reconciliation ecology and the future of species diversity. Oryx. 2003;37(2):194–205. [Google Scholar]
- 96.Phalan B, Onial M, Balmford A, Green RE. Reconciling food production and biodiversity conservation: Land sharing and land sparing compared. Science. 2011;333(6047):1289–1291. doi: 10.1126/science.1208742. [DOI] [PubMed] [Google Scholar]
- 97.Imhoff ML, et al. Global patterns in human consumption of net primary production. Nature. 2004;429(6994):870–873. doi: 10.1038/nature02619. [DOI] [PubMed] [Google Scholar]
- 98.Cerqueira Y, et al. Ecosystem services: The opportunities of rewilding in Europe. In: Pereira HM, Navarro LM, editors. Rewilding European Landscapes. Springer; Heidelberg: 2015. pp. 47–64. [Google Scholar]
- 99.Hobbs RJ, Higgs E, Harris JA. Novel ecosystems: Implications for conservation and restoration. Trends Ecol Evol. 2009;24(11):599–605. doi: 10.1016/j.tree.2009.05.012. [DOI] [PubMed] [Google Scholar]
- 100.Bowman D. Conservation: Bring elephants to Australia? Nature. 2012;482(7383):30. doi: 10.1038/482030a. [DOI] [PubMed] [Google Scholar]
- 101.Milner JM, Van Beest FM, Schmidt KT, Brook RK, Storaas T. To feed or not to feed? Evidence of the intended and unintended effects of feeding wild ungulates. J Wildl Manage. 2014;78(8):1322–1334. [Google Scholar]
- 102.Tanentzap AJ, Kirby KJ, Goldberg EE. Slow responses of ecosystems to reductions in deer (Cervidae) populations and strategies for achieving recovery. For Ecol Manage. 2012;264:159–166. [Google Scholar]
- 103.Intergovernmental Panel on Climate Change . Climate Change 2013: The Physical Science Basis. Cambridge Univ Press; Cambridge, UK: 2013. [Google Scholar]
- 104.Post E, Pedersen C. Opposing plant community responses to warming with and without herbivores. Proc Natl Acad Sci USA. 2008;105(34):12353–12358. doi: 10.1073/pnas.0802421105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Tanentzap AJ, Coomes DA. Carbon storage in terrestrial ecosystems: do browsing and grazing herbivores matter? Biol Rev Camb Philos Soc. 2012;87(1):72–94. doi: 10.1111/j.1469-185X.2011.00185.x. [DOI] [PubMed] [Google Scholar]
- 106.IUCN/SSC . Guidelines for Reintroductions and Other Conservation Translocations. Version 1.0. IUCN Species Survival Commission; Gland, Switzerland: 2013. [Google Scholar]
- 107.Burney DA, Juvik JO, Burney LP, Diagne T. Can unwanted suburban tortoises rescue native Hawaiian plants? Tortoise. 2012;1(1):104–115. [Google Scholar]
- 108.Seddon PJ, Moehrenschlager A, Ewen J. Reintroducing resurrected species: Selecting DeExtinction candidates. Trends Ecol Evol. 2014;29(3):140–147. doi: 10.1016/j.tree.2014.01.007. [DOI] [PubMed] [Google Scholar]
- 109.Redford KH, Adams W, Mace GM. Synthetic biology and conservation of nature: Wicked problems and wicked solutions. PLoS Biol. 2013;11(4):e1001530. doi: 10.1371/journal.pbio.1001530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Donlan J. De-extinction in a crisis discipline. Front Biogeogr. 2014;6(1):25–28. [Google Scholar]
- 111.Peterken GF. Habitat conservation priorities in British and European woodlands. Biol Conserv. 1977;11(3):223–236. [Google Scholar]
- 112.Faurby S, Svenning J-C. A species-level phylogeny of all extant and late Quaternary extinct mammals using a novel heuristic-hierarchical Bayesian approach. Mol Phylogenet Evol. 2015;84:14–26. doi: 10.1016/j.ympev.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 113.Faurby S, Svenning J-C. Historic and prehistoric human-driven extinctions have reshaped global mammal diversity patterns. Divers Distrib. 2015 doi: 10.1111/ddi.12369. [DOI] [Google Scholar]
- 114.Owen-Smith N. Contrasts in the large herbivore faunas of the southern continents in the late Pleistocene and the ecological implications for human origins. J Biogeogr. 2013;40(7):1215–1224. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.