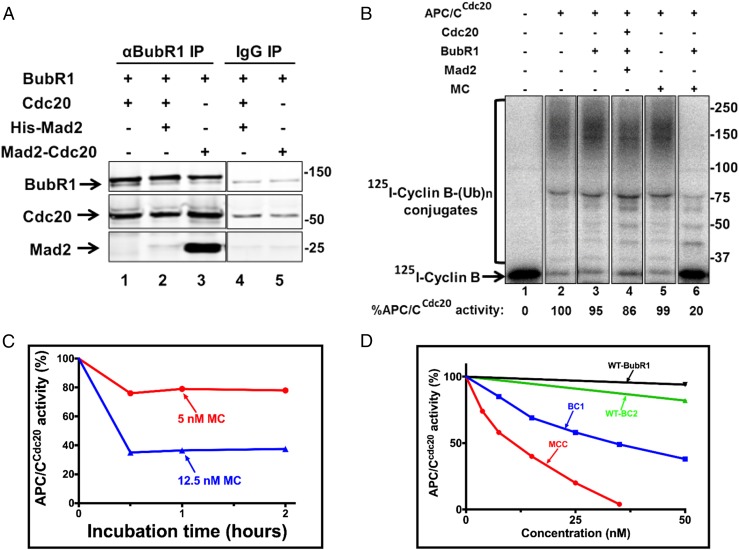
Fig. 2.
The Mad2-Cdc20 subcomplex is a preferred precursor for MCC formation, but does not act catalytically to produce an inhibitor of APC/C activity. (A) The Mad2-Cdc20 subcomplex is a preferred substrate for MCC assembly. Reaction mixtures contained in the volume of 20 µL: 50 Mm Tris⋅HCl (pH 7.6), 100 mM NaCl, 5 mg/mL BSA, 1 mM DTT, and 10% (vol/vol) glycerol. The indicated additions were at 150 nM. Following incubation at 23 °C for 60 min, samples were subjected to immunoprecipitation with anti-BubR1 as described in Materials and Methods. Sham treatment was under similar conditions with nonimmune rabbit IgG. Samples of immunoprecipitates were subjected to immunoblotting for the indicated proteins as described in Materials and Methods. Numbers on the right side indicate the migration positions of marker proteins (kDa). (B) MCC assembled from the Mad2-Cdc20 subcomplex and BubR1 in vitro preferentially inhibits APC/CCdc20. APC/CCdc20 bound to anti-Cdc27 beads was prepared as described in Materials and Methods. A total of 100 nM of the indicated checkpoint proteins was incubated for 1 h at 23 °C, followed by the addition of APC/Cdc20 and further incubation with shaking (1,400 × g) for 1 h at 23 °C. Subsequently, beads were washed three times with Buffer A and were subjected to a cyclin-B ubiquitin ligation assay as described in Materials and Methods. Finally, samples were subjected to SDS/PAGE and radioautography as described in Materials and Methods. The results indicated at the bottom are expressed as the percentage of I125-cyclin B-(Ub)n conjugates formed relative to APC/C activity with 100 nM Cdc20. Numbers on the right indicate the migration position of marker proteins (kDa). MC, Mad2-Cdc20. (C) Limiting amounts of Mad2-Cdc20 do not act catalytically to stimulate the formation of an APC/C inhibitor. BubR1 and Cdc20 (300 nM, each) were incubated with the indicated concentrations of Mad-Cdc20 (MC) for 1 or 2 h. Subsequently, mixtures were added to immunopurified APC/CCdc20, and cyclin ubiquitylation activity was assayed as described in B. Results are expressed as the percentage of APC/CCdc20 activity without MC at time 0. (D) Comparison of the effectiveness of BC-1, BC-2, and MCC on the inhibition of APC/CCdc20 activity. Purified MCC and subcomplexes, at the concentrations indicated, were incubated with APC/CCd20, and samples were subjected to a cyclin-B ubiquitin ligation assay as described in B. Results are expressed as the percentage of cyclin–ubiquitin conjugates formed relative to APC/CCdc20 activity without additions.