Abstract
Angiogenesis and lymphangiogenesis often occur in response to tissue injury or in the presence of pathology (e.g. cancer), and it is these types of environments in which macrophages are activated and increased in number. Moreover, the blood vascular microcirculation and the lymphatic circulation serve as the conduits for entry and exit for monocyte-derived macrophages in nearly every tissue and organ. Macrophages both affect and are affected by the vessels through which they travel. Therefore, it is not surprising that examination of macrophage behaviors in both angiogenesis and lymphangiogenesis has yielded interesting observations that suggest macrophages may be key regulators of these complex growth and remodeling processes. In this review, we will take a closer look at macrophages through the lens of angiogenesis and lymphangiogenesis, examining how their dynamic behaviors may regulate vessel sprouting and function. We present macrophages as a cellular link that spatially and temporally connects angiogenesis with lymphangiogenesis, in both physiological growth and in pathological adaptations, such as tumorigenesis. As such, attempts to therapeutically target macrophages in order to affect these processes may be particularly effective, and studying macrophages in both settings will accelerate the field’s understanding of this important cell type in health and disease.
Keywords: monocyte, macrophage, angiogenesis, lymphangiogenesis, endothelial cell, pericyte, tumor-associated macrophage
INTRODUCTION
Macrophages were discovered one hundred and thirty years ago [276], and they have long been regarded as an important part of the body’s immune system for their phagocytic capabilities. For the last thirty years, we have appreciated their ability to secrete growth factors, chemokines, and extracellular matrix (ECM)-modifying enzymes that modify both the structure and function of the local environment [83,244,269]. However, only recently and because of technological advances in imaging, in vivo cell tracking, and flow cytometry, have we begun to learn about their phenotypic flexibility and the broad range of dynamic cell behaviors that macrophages exhibit during development, following injury, and in disease. Angiogenesis and lymphangiogenesis are prominent in these tissue remodeling settings, so it is perhaps not surprising that roles for -- and questions about -- macrophage identity, phenotype, and function in the context of blood vessel and lymphatic vessel growth are surfacing in the literature at a rapid rate. Amidst this “renaissance of the macrophage” our review aims to summarize the current understanding of macrophages, with a specific emphasis on their presumptive common roles in angiogenesis and lymphangiogenesis (Figure 1). Our focus on the current literature attempts to highlight some of the ongoing debates and unresolved questions that may have therapeutic relevance. First, we discuss macrophage origin and phenotypic diversity from an immunology perspective. Then, we highlight different macrophage behaviors and their interactions with vascular cells that have been shown to be important in regulating angiogenesis and lymphangiogenesis. Throughout our review, we point out some of the newly identified roles for macrophages, and we speculate about how macrophages could provide a mechanistic bridge between these two vessel remodeling processes. Finally, we discuss strategies for therapeutically targeting macrophages in the context of disease, and cancer in particular.
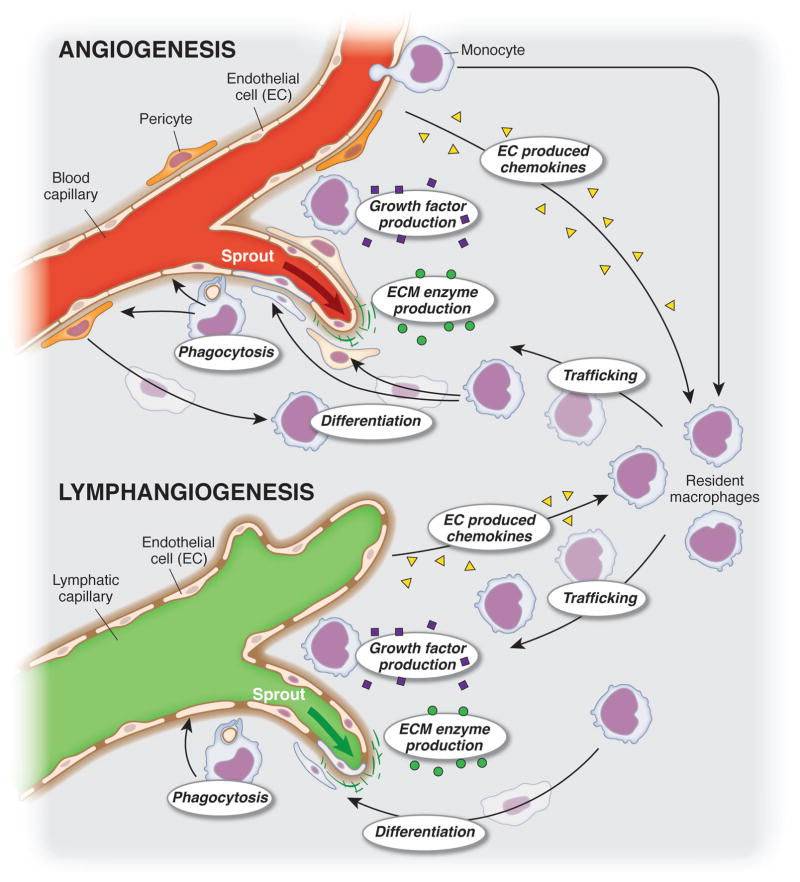
Figure 1. Macrophage dynamics during angiogenesis and lymphangiogenesis.
Macrophages exhibit a variety of dynamic cell behaviors, including phagocytosis, trafficking, differentiation, proliferation, and secretion of growth factors, enzymes, and chemokines, that are potentially important to the growth and remodeling of the microvasculature during angiogenesis and lymphangiogenesis. The common dynamics implicates macrophages as an attractive cellular target for therapies pro- and against both processes a cellular link that can be targeted.
MACROPHAGE ORIGINS, LINEAGES, AND PHENOTYPIC DIVERSITY
Macrophages are versatile cells that have phenotypic diversity and carry out complex functions in disease and homeostasis (Figure 2, Table 1). The literature suggests that different subpopulations of macrophages play key roles in directing the innate immune response during developmental processes, as well as in initiation of injury, resolution of injury, and in chronic inflammatory conditions [5,65]. Furthermore, tissue resident macrophages in many organs have unique gene expression profiles [22,158] and fulfill special roles necessary for healthy function of the organ [122]. Technological breakthroughs in antibody design, lineage tracing, flow cytometry, and in vivo imaging have enabled immune cell phenotyping at an unprecedented level of detail. This has led to the identification of different sub-sets of macrophages and a deeper understanding of their diverse origins. In order to understand – and be able to question – the role of macrophages in angiogenesis and lymphangiogenesis, it is first important to summarize their origins, lineages, and phenotypic diversity.
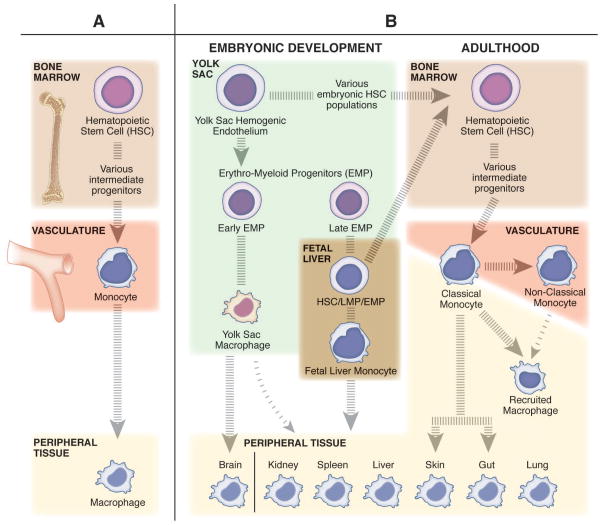
Figure 2. The diverse origins of macrophages and monocytes.
(A) The classic belief that tissue resident macrophages are populated from monocytes through adult hematopoiesis. In the bone marrow, hematopoietic stem cells (HSCs) differentiate into monocytes through a series of intermediate progenitors, extravasate from the vasculature, and differentiate into macrophages to maintain the tissue resident niches. (B) The more contemporary view that tissue resident macrophages undergo self-renewal, and have a distinct embryonic origin sourced from populations of erythro-myeloid progenitors (EMPs) derived from the yolk sac hemogenic endothelium. The early population of EMPs differentiate into yolk sac macrophages and then migrate to the tissues and contribute to the tissue resident macrophage population. The late population of EMPs gives rise to fetal liver monocytes through an unknown intermediate embryonic progenitor cell type (maybe EMP, HSC, or lympho-myeloid progenitor (LMP)). Fetal liver monocytes then go on to seed the tissue resident macrophage populations for all peripheral tissues other than the brain. Only the skin and gut resident macrophage populations are recognized to be replenished in homeostatic conditions by bone marrow derived cells, specifically classical monocytes. Classical monocytes (inflammatory monocytes) patrol the peripheral tissues in homeostatic conditions, while nonclassical (anti-inflammatory monocytes) surveil the vasculature. In inflammatory conditions, classical monocytes, and to a lesser extent nonclassical monocytes, can leave the vasculature and differentiate into recruited macrophages to transiently regulate the immune response.
Table 1.
Subpopulations of macrophages and monocytes relevant to angiogenesis and lymphangiogenesis
| Name | Tissue Compartment | Origin in Adult | Notable Functions and Characteristics | Reference |
|---|---|---|---|---|
| Classical Monocyte | Bloodstream, Spleen, Tissue | Adult Bone Marrow | Patrol peripheral tissue in homeostasis. | [303] |
| Intermediate Monocyte | Bloodstream, Spleen | Circulation | High expression of Tie2, VEGF-A and MMP-9. | [316] |
| Nonclassical Monocyte | Bloodstream, Spleen | Circulation | Patrol bloodstream for immune surveillance. | [303] |
| Recruited Macrophage | All Peripheral Tissues | Adult Bone Marrow | Transiently bolster immune response and recovery in peripheral tissues during inflammatory conditions. | [93,303] |
| Perivascular Macrophage | All Peripheral Tissue | Adult Bone Marrow (?) | Mediate neutrophil recruitment during inflammation. | [2,100] |
| Kupffer Cells | Liver | Fetal Liver | Prevent inflammatory response of nonpathogenic stimuli such as blood borne food and commensal bacteria antigens. | [65] |
| Alveolar Macrophages | Lung | Fetal Liver | Clearance of excess and degraded alveolar surfactant layer components: insures optimum gas exchange through alveoli. | [70,74] |
| Red Pulp Macrophages | Spleen | Adult Bone Marrow | Phagocytosis of injured and senescent erythrocytes and blood-borne particulates. | [74,156] |
| Marginal Zone Macrophages | Spleen | Unknown | Early infection response by trapping blood borne antigens, rapidly removing them from circulation. | [4,74] |
| Intestinal Macrophages | Intestines | Adult Bone Marrow | Regulates peristalsis of the colon. | [74,190] |
| Dermal Macrophages | Skin | Adult Bone Marrow | Specialized roles in scavenging and killing microbes. Unable to migrate to and activate T cells. | [74,275] |
| Cardiac Macrophages | Heart | Variousa | Protect heart muscle from hypertensive stress response. | [74,89] |
| Kidney Macrophages | Kidney | Adult Bone Marrow (?) | Regulation of tubule cell apoptosis through nitric oxide. | [74,150] |
| Peritoneal Macrophages | Peritoneal Cavity | Fetal Liver | Massive disappearance of these cells under inflammatory conditions (function unknown). | [37] |
| Microglia | Brain | Yolk Sac | Necessary for neuronal pruning during development, and important for neuronal homeostasis, synaptic remodeling, regulating blood brain barrier. | [198] |
| Adipose Associated Macrophages | Adipose Tissue | Unknown | Regulate insulin sensitivity and body temperature. | [58,199] |
| Osteoclast | Bone Marrow | Adult Bone Marrow | Bone remodeling, support for hematopoiesis. | [122] |
Heart macrophages are split into two groups: CCR2- macrophages originate from yolk sack and fetal liver, CCR2+ macrophages originate from adult bone marrow.
Abbreviations: CCR2: chemokine (C-C motif) receptor 2, Tie2: receptor tyrosine kinase of the Tie family, VEGF: vascular endothelial growth factor family, MMP-9: matrix metaolprotinase-9.
Macrophage origins
Since the identification of the mononuclear phagocytic system in the 1960s, it was believed that macrophages found in the peripheral tissues were continuously replenished by hematopoietic stem cells in the bone marrow that differentiate through a series of intermediate progenitor cells to monocytes (Figure 2A) [283]. Monocytes are a phagocytic white blood cell of the innate immune system that ingest pathogens and cellular debris, present antigen to T cells, and have the capacity to differentiate into macrophages when they extravasate from the vasculature [222]. However, emerging evidence suggests that macrophages have alternative origins, wherein various tissue-resident macrophage subpopulations undergo self-renewal within their tissues. This may have been overlooked until recently because of the reliance on bone marrow chimeric models to elucidate macrophage ontogeny [282,304]. In bone marrow chimeric models, mice are exposed to gamma irradiation, which destroys bone marrow stem cells. Then, irradiated mice receive an injection of donor stem cells that are typically modified to express a fluorescent marker, such as GFP, and reconstitute the bone marrow so as to enable visual tracking of monocyte-derived macrophages based on their fluorescence [107]. A limitation with this approach, however, is that the radiation used to deplete stem cells in the bone marrow also inflicts whole body injury, causing increased vascular permeability [69,140] and other non-physiologic behaviors [146,170]. This may artificially inflate the number of fluorescent monocyte-derived macrophages that are observed in injured tissues, especially in studies of the central nervous system [146,176,215,216,258]. Hence, the recent use of cell-type specific, endogenous fluorescent lineage reporters is revealing, for the first time, the abundance of tissue-resident macrophages that are not monocyte derived [105] but that instead originate from embryonic tissues, as discussed below.
Macrophage lineages
Macrophages can generally be divided into three groups: 1) recruited macrophages, 2) tissue-resident macrophages, and 3) perivascular macrophages. Recruited macrophages, also called “adult monocyte derived macrophages” or “infiltrating macrophages”, only transiently remain in the tissue compartment during inflammation to aid with the immune response [5], and then are hypothesized to either undergo apoptosis [122], emigrate from the tissue to the lymph nodes [253], reenter the bloodstream [96], or in some cases differentiate into a tissue-resident macrophage [122]. Recruited macrophages are derived primarily from classical monocytes (also called inflammatory monocytes), the dominant monocyte population that during homeostatic conditions can extravasate from blood vessels and patrol peripheral tissues with minimal differentiation [127]. Classical monocytes can differentiate to nonclassical monocytes (anti-inflammatory monocytes), a small subpopulation that remains in the bloodstream for immune surveillance and patrolling the vasculature [277]. During inflammatory conditions, classical monocytes are thought to often favor differentiating into recruited macrophages with an inflammatory phenotype, while nonclassical monocytes favor an anti-inflammatory phenotype [303]. Recently, a third subpopulation of monocytes has been proposed, intermediate monocytes, that have highly elevated expression of the angiogenic Tie-2 receptor and secretion of angiogenic cytokines like VEGF-A and MMP-9 [316]. While little is known about this subgroup, even whether it represents a transient switch between other subpopulations, one study found that intermediate monocytes were the only blood mononuclear cell that could induce capillary formation in a model of tumor angiogenesis, making this monocyte population a particularly interesting effector cell to examine for future investigations [60].
The majority of tissue resident macrophage populations are now recognized to be of embryonic origin and renew themselves primarily through mechanisms that are independent of hematopoiesis [105]. Tissue resident macrophages arise from the yolk sac hemogenic endothelium, a subpopulation of developmental pluripotent endothelial cells that exist in early embryogenesis [310]. These cells give rise to erythro-myeloid progenitors (EMPs), which are split into two subpopulations based on time of formation (Figure 2B). Early EMPs give rise to yolk sack macrophages that eventually populate the brain to become microglia, the tissue resident macrophage for that organ [114]. Late EMPs migrate to the fetal liver and produce fetal liver monocytes, which enter the bloodstream, migrate to the peripheral tissues, and differentiate into the various tissue resident macrophages. Notable exceptions are the resident macrophage populations of the skin and gut, which are replenished continuously from classical monocytes [175,189]. While studies have shown that some subpopulations of recruited macrophages, predominantly of anti-inflammatory phenotype, can take up long term residence in tissues, there is evidence in some tissues that they do not transition to tissue resident macrophages and perform their specialized functions [5]. It is important to note that with the recent call to stringently classify macrophage subpopulations based on ontogeny [101], microglia will likely receive a distinct classification that separates them from all other tissue resident macrophages if their unique lineage is verified.
In both quiescent and inflammatory conditions, perivascular macrophages closely associate with the post-capillary venules in peripheral organs including skin, muscles, heart [98], central nervous system [149,297]. Whether or not this macrophage subpopulation exists in other tissues during quiescence is not known. Several studies indicate that perivascular macrophages play a key role in immune cell recruitment and extravasation [2,106]. However, the uniqueness of perivascular macrophages is unclear because an exclusive lineage marker has yet to be identified for this subpopulation and many studies simply merge this group with monocyte derived macrophages or tissue resident macrophages [57,100]. Perivascular macrophages are hypothesized to originate from adult monocytes [23,217], but this evidence is based on bone marrow chimera experiments, which, as discussed above, can create a nonphysiologic environment [140]. Regardless of whether this subgroup is derived from the tissue resident niche or from monocytes, we believe that this population should remain distinct from other tissue-resident macrophages and from recruited monocyte-derived macrophages: perivascular macrophages possess a unique morphology and localization along the vasculature compared to other tissue resident macrophages. Therefore, they may experience different metabolic stimuli and cytokine profiles as a result of being more proximal to the circulating blood pool. Moreover, perivascular macrophages in quiescent tissues may be uniquely pre-conditioned by the tissue environment in which they have resided in contrast to the circulating monocyte-derived macrophages that are recruited during inflammation [158]. Importantly, the potential presence of perivascular macrophages in the same local environments (or “niches”) as tissue resident and inflammatory macrophage sub-types motivates future investigations aimed at determining whether the three sub-types are more similar than different when it comes to their functionality in the tissue -- especially in developing or injured tissues, such as those where angiogenesis and/or lymphangiogenesis are occurring.
Macrophage phenotypic diversity
Macrophages exhibit a vast and dynamic range of different phenotypes. Within the field of immunology, macrophage classification schemes have emerged and are undergoing redefinition [178], with several new, competing schemes proposed to incorporate recent biological insights. The prevailing macrophage phenotype classification scheme is inspired by T helper cell nomenclature. Classically activated macrophages, known as the M1 phenotype, are stimulated by IFN-γ, TNF-α, and LPS, and provoke secretion of cytotoxic agents, such as nitric oxide, and pro-inflammatory cytokines, including IL-1, IL-6, IL-12, IL-23, and TNF-α [178]. Alternatively activated macrophages, known as the M2 phenotype, are stimulated by IL-4 and IL-10, and these cells secrete the growth promoting molecule, orthinine [183], as well as anti-inflammatory cytokines that promote angiogenesis, such as IL-10 and TGF-β [235]. In terms of their metabolism, M1 macrophages obtain energy primarily through glycolysis and metabolize arginine though nitric oxide synthase, while M2 macrophages obtain energy through oxidative pathways and metabolize arginine through arginase to fuel their longer-term functions associated with inflammation resolution [90,183,224].
An expanded version of this classification scheme renamed M2 to “M2a”, and added several additional groups, including: M2b, which is characterized by immune complex formation and toll-like receptor activation, M2c, which represents deactivated macrophages that suppress proinflammatory cytokines, and M2d, which represents a regulatory macrophage [236] that is often grouped with tumor associated macrophages (TAMs) [27,51,104]. However, this classification scheme has possible nomenclature pitfalls, such as the fact that M2 macrophages behave as pro-inflammatory macrophages in certain situations [21,116] and do not necessary exhibit clear-cut activation states in vivo [178].
An alternative classification scheme proposes a three-category “color wheel” of macrophage activation that categorizes the cells according to specific functions they perform: classically activated macrophages, wound-healing macrophages, and regulatory macrophages [187]. Yet another classification scheme parses macrophage phenotype based on in vitro stimulation of macrophages using individual cytokines and paired cytokine combinations [193]. It may also be possible to characterize macrophages in vivo based on the paracrine factors that they secrete, which can be determined using fluorescent in situ hybridization (FISH) probes against mRNA transcripts for cytokines [309], such as IL-1 and IL-10. FISH can be multiplexed with standard immunostaining, and conducted on whole mount or tissue sections [214].
While each macrophage classification scheme is unique, we attempt to relate them to one another by providing a suggested mapping of macrophage subpopulations and activation states across these different classification schemes (Table 2). It is unclear which of these macrophage classification schemes will gain the most widespread acceptance in the long term. In the meantime, we recommend that studies evaluating macrophage phenotype should: 1) use panels of expression markers that are consistent with the literature, 2) reflect on the latest insights in immunology to aid in the interpretation of findings, and 3) consider the possibility that phenotype determinations may be accurate only for transient windows of time, especially in settings of dynamic tissue remodeling involving angiogenesis and/or lymphangiogenesis.
Table 2.
Mapping of M1/M2 macrophage polarization states to other published macrophage classification schemes.
| Scheme | Phenotype | ||||
|---|---|---|---|---|---|
|
| |||||
| Bipolar |
|
||||
|
| |||||
| Extended Bipolar | M1 | M2b | M2c | M2a | M2d |
| Surface Markers | CD80 | CD86 | CD163 | CD206 | CD163 |
| CD86 | MHCII | TLR1 | CD163 | ||
| TLR2 | TLR8 | MHCII | |||
| TLR4 | IL-1R II | ||||
| MHCII | Fizz1 | ||||
|
| |||||
| Secreted Cytokines | TNF-α | IL-1 | IL-10 | IL-10 | IL-10 |
| IL-1β | IL-6 | TGF-β | TGF-β | IL-12 | |
| IL-6 | IL-10 | IL-1rα | TNF-α | ||
| IL-12 | TNF-α | TGF-β | |||
|
| |||||
| Stimulus Based |
M(IFN-γ) M(LPS+INF-γ) |
M(LPS+INF-γ) M(LPS) |
M(−) M(GC) |
M(GC+TGF-β) M(IL-10) |
M(Ic) M(IL-4) |
|
| |||||
| Color Wheel | Inflammatory | Regulatory | Wound Healing | ||
|
| |||||
| Secreted Cytokines | TNF-α | IL-10 | CCL18 | ||
| iNOS | SPHK1 | CCL18 | |||
| CCL15 | LIGHT | CCL18 | |||
| CCL20 | CCL1 | CCL18 | |||
Abbreviations: CD80: cluster of differentiation 80, CD86: cluster of differentiation 86, TLR2: toll-like receptor 2, TLR4: toll-like receptor 4, TNF-α: tumor necrosis factor α, IL-1β: interleukin-1 beta, IL-6: interleukin-6, IL-12: interleukin-12, CD163: cluster of differentiation 163, TLR1: toll like receptor 1, TLR8: toll-like receptor 8, IL-10: interleukin-10, TGF-β: transforming growth factor beta, CD86: cluster of differentiation 86, MHCII: major histocompatibility complex II, SR: scavenger receptor CD206: cluster of differentiation 206, IL-1: interleukin-1, IL-1ra: interleukin-1 receptor a, CD14: IL-12: interleukin-12, iNOS: inducible nitric oxide synthase, CCL15: chemokine (c-c motif) ligand 15, CCL20: Chemokine (c-c motif) ligand 20, SPHK1: sphingosine kinase 1, LIGHT: TNF superfamily 14, CCL1: chemokine (C-C motif) ligand 1, CCL18: chemokine (c-c motif) ligand 18, FACTOR XIII-A: fibrin stabilizing factor, RELMα: Resistin-Like Molecules alpha, CCL17: chemokine (c-c motif) ligand 17.
MACROPHAGE DYNAMICS IN ANGIOGENESIS
Trafficking of monocyte-derived macrophages in angiogenesis
Recruited macrophages can be derived from monocytes that are activated in the bone marrow by chemokines like MCP-1 to enter the bloodstream and traffic to sites of tissue injury [252]. This process follows a step-wise sequence of events that is characteristic for most leukocytes, which has been termed the “leukocyte adhesion cascade” [165]. Many of the hematopoietic cytokines (e.g. GM-CSF, [91]) and cell adhesion molecules (e.g. P-selectin, [18]) implicated in monocyte trafficking have historically been associated with circulating “progenitor cell” trafficking during injury-induced vascular growth and remodeling. Indeed, shared trafficking mechanisms between bone marrow-derived, pro-angiogenic “progenitor cells”, such as the endothelial progenitor cells (EPCs) identified in the late 1990’s [15], may have both contributed to the confusion and helped to resolve the controversy over EPC identity. Critical reassessment during the last five years has suggested that EPCs are linked to the plasticity of monocyte–derived macrophages [75], and their participation in adult angiogenesis is more accurately described by macrophage dynamics, which will be discussed in more detail below.
The classical view is that monocyte trafficking into injured tissue occurs from post-capillary venules, and recent studies have confirmed that post-capillary venules are important sites for monocyte entry into the tissue during microvascular growth and remodeling [32]. Post-capillary venules have also long been regarded as the vessels in the microcirculation that give rise to new capillaries during angiogenesis [192,212]. Moreover, it has been suggested that monocytes preferentially transmigrate through post-capillary venules at locations where ECM protein levels in the basement membrane are decreased [293]. Because the early steps of capillary sprouting angiogenesis are associated with ECM remodeling in the basement membrane [237], and monocyte-derived macrophages are known to secrete ECM modifying proteins (e.g. MMP-9) that may further enhance a tissue’s ability to support capillary sprouting [62], the spatial-temporal relationships between monocyte-derived macrophage trafficking and angiogenesis in post-capillary venules may have important consequence. Indeed, over twenty-five years ago, it was suggested that macrophage influx and angiogenesis were spatially correlated and likely causally intertwined in tumorigenesis [196], although the details of this interrelationship are still being clarified.
Images of macrophages at single time-points in different remodeling microvascular networks (Figure 3) allude to how these cells traffic to tissues and interact with vascular cells following extravasation. Micrographs such as these also motivate the need for dynamic imaging approaches that capture the time-course of monocyte-derived macrophage trafficking. What if there are other routes of monocyte extravasation during angiogenesis, for example, through new capillary sprouts [85]? Could alternative trafficking dynamics offer new insights about the role of monocytes and macrophages in endothelial tip-cell specification? Or is it possible that monocyte-derived macrophages that have trafficked to/through capillary sprouts differentiate directly into blood endothelial cells because of their spatial positioning in the neovessel wall? These are just a few of the currently unanswered questions that may benefit from the new experimental tools and models described in the final section of this review. Importantly, at the present time the dynamics of macrophages in tissues (including tumors) are incompletely characterized, and there is evidence to support that macrophage flux, both into and out of tissues, can be affected by the tissue environment and whether there is an acute or chronic disruption to tissue homeostasis.
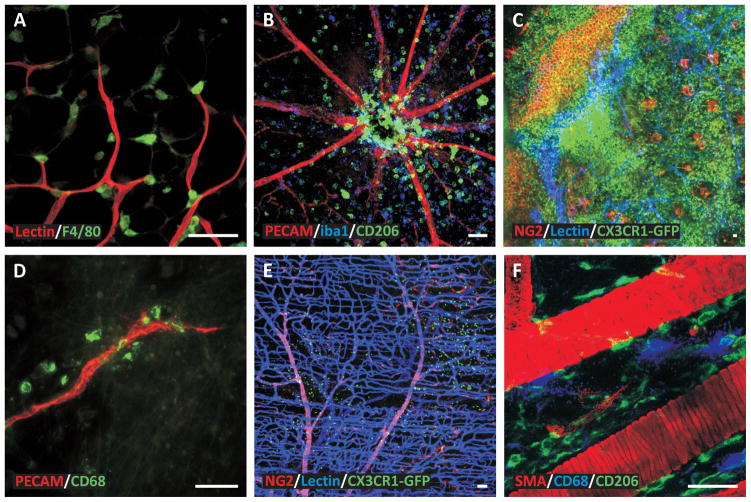
Figure 3. Examples of macrophage spatial distributions during angiogenesis in various tissues.
The heterogeneity in macrophage localization emphasizes the need to identify how and why macrophages are recruited to specific locations within microvascular networks. A) Mouse adipose tissue stimulated by a ligation in a feeding arter (Red: Blood vessels, Green: Macrophages). B) Mouse retina stimulated by IL-1β. (Red: Blood vessels, Blue: M2a Macrophages, Green: Macrophages). C) Mouse ear tissue stimulated by a burn injury (Red: Perivascular cells, Blue: Blood vessels, Green: Macrophages). D) Rat mesentery tissue stimulated by chronic hypoxia (Red: Blood vessels, Green: Macrophages). E) Mouse spinotrapezius muscle (Red: Perivascular cells, Blue: Blood vessels, Green: Macrophages). F) Mouse spinotrapezius muscle (Red: Smooth muscle cells, Blue: Mast cells, Green: Macrophages). Scale bars = 50 microns.
Paracrine roles for macrophages in angiogenesis
Macrophages have been shown to secrete a variety of pro- and anti-angiogenic growth factors (Table 3). Secretion of VEGF-A by monocyte-derived macrophages, in particular, appears to be critical for inducing angiogenesis in tissue repair [266]. Using a CCR2-eGFP reporter mouse model, Willenborg et al. demonstrated that VEGF-A expression by monocyte-derived CCR2+Ly6C+ macrophages is required for the induction of capillary sprouting in the setting of skin wound healing [172,300]. In full-thickness cutaneous wounds made in CCR2 knockout mice, where the recruitment of Ly6C+ circulating blood monocytes was impaired, reduced VEGF-A expression levels in granulation tissue was accompanied by significantly decreased angiogenesis [280]. In this study, VEGF-A -expressing macrophages that were recruited to the wound site exhibited characteristics of M1 and M2 macrophage sub-sets, including expression of the proinflammatory mediators IL-6 and iNOS, as well as Tie-2, which has been shown to be expressed by tumor-associated, M2-like macrophages [300]. Moreover, evaluation of wounds in mice with myeloid cell–specific VEGF-A deletion revealed that macrophage-derived VEGF-A during the early stage of tissue growth was critical to achieving physiologic tissue vascularization and healing.
Table 3.
Pro- and anti-angiogenic/lymphangiogenic cytokines and other paracrine signals secreted by monocytes and macrophages
| Cytokine Name | Effect | Reference |
|---|---|---|
| Acidic Firbroblast Growth Factor (aFGF) | Pro-Angiogenic | [31,295] |
| Angiopoietin-2 (Ang2) | Pro-Angiogenic, Pro-Lymphangiogenic | [80,119,314] |
| Angiostatin | Anti-Angiogenic, Anti-Lymphangiogenic | [76,180,251] |
| Angiotensin converting enzyme (ACE) | Anti-Angiogenic | [278,287,306] |
| Angiotropin | Pro-Angiogenic | [112,113] |
| Basic Fibroblast Growth Factor (bFGF) | Pro-Angiogenic, Pro-Lymphangiogenic | [206,268] |
| Chemokine (C-C motif) Ligand 2 (CCL2) | Pro-Angiogenic, Pro-Lymphangiogenic | [63,133,262] |
| Epithelial Neutrophil Activating peptide-78 (ENA-78/CXCL5) | Pro-Angiogenic | [99,166] |
| Granulocyte colony stimulating factor (G-CSF) | Pro-Angiogenic, Anti-Angiogenic | [160,197,234,279] |
| Granulocyte-macrophage colony stimulating factor (GM-CSF) | Pro-Angiogenic, Pro-Lymphangiogenic | [157,254,313] |
| Insulin-like Growth Factor 1 (IGF-1) | Pro-Angiogenic, Pro-Lymphangiogenic | [28,268] |
| Insulin-like Growth Factor 2 (IGF-2) | Pro-Angiogenic, Pro-Lymphangiogenic | [28,267] |
| Interleukin-1 Alpha (IL-1α) | Pro-Angiogenic, Pro-Lymphangiogenic | [290,294] |
| Interleukin-1 Beta (IL-1β) | Pro-Angiogenic; Pro-Lymphangiogenic | [290,294] |
| Interleukin-10 (IL-10) | Anti-Angiogenic | [301] |
| Interleukin-19 (IL-19) | Pro-Angiogenic | [229] |
| Interleukin-20 (IL-20) | Pro-Angiogenic, Anti-Angiogenic, Pro-Lymphangiogenic | [103,110,117] |
| Interleukin-6 (IL-6) | Pro-Angiogenic, Pro-Lymphangiogenic | [34,77] |
| Macrophage-derived Endothelial Cell Inhibitor (MD-ECI) | Anti-Angiogenic | [26] |
| Matrix Metalloproteinase-2 (MMP-2) | Pro-Angiogenic, Pro-Lymphangiogenic | [64,246] |
| Matrix Metalloproteinase-9 (MMP-9) | Pro-Angiogenic, Pro-Lymphangiogenic | [25,64,308] |
| N-acetyl-Ser-Asp-Lys-Pro (Ac-SDKP) | Pro-Angiogenic | [195,265] |
| Platelet Derived Growth Factor (PDGF) | Pro-Angiogenic, Pro-Lymphangiogenic | [35,243] |
| Platelet-derived Endothelial Cell Growth Factor (PD-ECGF) | Pro-Angiogenic | [86] |
| Thrombospondin-1 (TSP-1) | Anti-Angiogenic, Anti-Lymphangiogenic | [53,84,159] |
| Thrombospondin-2 (TSP-2) | Anti-Angiogenic | [159,209] |
| Transforming Growth Factor-α (TGF-α) | Pro-Angiogenic | [268] |
| Transforming Growth Factor-β (TGF-β) | Pro-Angiogenic, Anti-Lymphangiogenic, Pro-Lymphangiogenic | [82,128,163,205] |
| Tumor Necrosis Factor Alpha (TNF-α) | Pro-Angiogenic, Pro-Lymphangiogenic | [162,227] |
| Urokinase Plasminogen Activator (uPA) | Pro-Angiogenic, Pro-Lymphangiogenic | [12,204] |
| Vascular Endothelial Growth Factor A xxx (VEGFAxxx) | Pro-Angiogenic, Pro-Lymphangiogenic | [52,201,301] |
| Vascular Endothelial Growth Factor A xxx b (VEGFAxxxb) | Anti-Angiogenic | [201] |
| Vascular Endothelial Growth Factor C | Pro-Angiogenic, Pro-Lymphangiogenic | [24,257] |
| Vascular Endothelial Growth Factor D | Pro-Angiogenic, Pro-Lymphangiogenic | [129,133,231] |
It has also been suggested that the polarization state of macrophages can affect the profile of growth factors that they secrete, so this may imply that different macrophage subset affect different stages of angiogenesis – from capillary sprout initiation to neovessel maturation [259]. By analyzing the expression of genes and the angiocrine secretion profiles of different subsets of macrophages, Spiller et al. showed that M1 macrophages express genes involved in the initiation of angiogenesis, including VEGF-A and FGF2. They also showed that M2a macrophages express and secrete high levels of PDGF-BB, while M2c macrophages secrete high levels of MMP-9. By analyzing macrophage infiltration and the extent of angiogenesis in collagen scaffolds that were implanted subcutaneously in a murine model for 10 days, these authors found that both M1 and M2 macrophages were required to achieve vascularization. Uncrosslinked collagen scaffolds were surrounded by a fibrous capsule containing predominantly M2 macrophages, and they underwent minimal angiogenesis. In contrast, glutaraldehyde-crosslinked collagen scaffolds recruited both M1 and M2 macrophages and experienced more robust angiogenesis. Based on the different growth factor secretion profiles and their apparent synergistic behaviors in bioimplant angiogenesis, the authors suggested that M1 macrophages stimulate capillary sprouting, while M2 macrophages may aid in vessel stabilization through pericyte recruitment.
Overall, these studies imply that tissue engineering and regenerative medicine strategies that take steps to control macrophage dynamics may achieve more effective angiogenesis, particularly in the context of bioimplant integration into the recipient tissue bed. Along these lines, the inclusion of monocyte and macrophage chemo-attractants in bioimplant designs has been pursued as a strategy to enhance macrophage recruitment and the induction of angiogenesis. In a recent study, co-delivery of CSF1 with VEGF-A in a hydrogel implant induced more robust angiogenesis and pericyte-recruitment to neovessels in the murine corneal pocket angiogenesis model than VEGF-A alone [118]. Such a finding exemplifies the value of elucidating the relative contribution of macrophages to angiogenesis versus other factors or cellular players. Interestingly, Kobyashi et al. demonstrated in 2002 that the direct injection of leukocytes induces angiogenesis, yet since this time more attention has been given to the use of various stem cell populations as cell-based stimulators of growth [13,50,182]. Hence, it is important to consider which cell type dominates in a dynamic growth and remodeling setting and how different cell types influence one another (e.g. mesenchymal stem cells and macrophages). This point was recently highlighted by a study showing that the therapeutic effects of adipose-derived progenitor cells was dependent on the presence of CD11b+ macrophages [186].
Macrophage interactions with endothelial cells, pericytes, and vascular smooth muscle cells
Macrophages engage in a number of heterotypic cell-to-cell interactions with other cell types, including endothelial cells, pericytes, and vascular smooth muscle cells, and these interactions help to regulate angiogenesis during embryonic development and in adult responses to injury. Macrophages were first characterized by their phagocytic capabilities, so it is not surprising that they have been shown to orchestrate neovessel pruning during the developmental maturation of microvessel networks. Macrophage pruning of vessels via phagocytosis of endothelial cell and pericytes has been identified in a number of different vascular patterning processes during development and post-natal remodeling. During fetal organogenesis of the testes, for example, macrophages arise from primitive yolk sac-derived hematopoietic progenitors, and nearly all of them express CD206, a marker consistent with an M2 macrophage polarization state [61]. Macrophage depletion in this tissue results in disrupted vascular patterning due to inadequate to vascular pruning -- a macrophage behavior that is also known to be important in the murine postnatal eye during postnatal pruning of the hyaloid and pupillary membrane vasculatures [171,213]. In adulthood, macrophage phagocytic behaviors may be important in pathological microvascular remodeling. In rats made diabetic by streptozotocin injection, CCL2 levels were upregulated and there was a two-fold increase in the number of CX3CR1+/CD11b+ macrophages in the retina [223]. Whether the elevated levels of macrophages observed in this diabetic rodent model portend phagocytosis as a mechanism underpinning retinal vessel pruning and edema in patients with diabetic retinopathy remains to be determined.
In addition to their phagocytic capabilities, macrophages have been shown to make cell-to-cell contacts with endothelial cells in an orientation that “bridges” two or more angiogenic sprouts. In studies of hindbrain development in mouse and zebrafish, macrophage bridging of endothelial cells enhanced endothelial cell tip cell fusion, or anastomosis [78]. Similarly, in the developing retina, tissue-resident macrophages, or microglia, have also been identified associating with endothelial tip cells. Genetic ablation of microglia in the Csf1op/op mouse has been shown to result in a sparser retinal vascular network with less branching [153], and in one study also resulted in endothelial sprouts with fewer filopodia [239]. To what extent macrophage bridging enacts control over endothelial cell sprout anastomosis during angiogenesis in the adult, and whether the effects of macrophage bridging are mediated by paracrine and/or juxtracrine signaling with endothelial cells, have yet to be determined.
Macrophages have been shown to interact directly with microvascular pericytes during tissue injury in response to pericyte-derived chemokines, such as MIF [264]. In the inflamed mouse ear stimulated by TNF or LPS, dynamic intravital two-photon microscopy has revealed that pericytes instruct macrophages with pattern-recognition and motility programs via ICAM-1 dependent contact after they have extravasated from post-capillary venules [264]. Very recent evidence also suggests that the recruitment of TAMs promotes pericyte-endothelial interactions in the brain, and in the absence of macrophages, pericytes lose their ability to associate with endothelial cells [307]. Together, these studies imply that pericytes and macrophages have two-way communication with one another that could be important in directing angiogenesis and microvascular remodeling.
Macrophages also affect vascular smooth muscle cell behaviors that are relevant to growth and remodeling of the microcirculation. For example, monocyte-derived macrophages play an important role in ligation-induced arteriogenesis by stimulating collateral artery diameter enlargement through smooth muscle cell hypertrophy and proliferation [32]. Recent studies by our group and by others have further suggested that macrophage recruitment and polarization may be a viable therapeutic target for enhancing collateral microvessel growth in settings of tissue ischemia [16,33,39,200].
Evidence for macrophage differentiation into vascular cells
An emerging body of evidence alludes to the fact that macrophages may possess the ability to trans-differentiate into vascular wall cells, including vascular smooth muscle cells, pericytes, and endothelial cells. Recent work by Shankman et al., has suggested that vascular smooth muscle cells residing in the medial layer of large vessels can give rise to macrophages and mesenchymal stem cells in response to injury [250]. Vascular smooth muscle cell-specific conditional knockout of Krüppel-like factor 4 (Klf4) reduced the numbers of SMC-derived macrophage and mesenchymal stem cells, as well as the size of atherosclerotic lesions. Whether the converse is true – i.e. that macrophages possess the ability to differentiate into SMCs -- has sparked recent debate [273]. And, whether the transdifferentiation of macrophages to SMCs or SMCs to macrophages is a phenomenon of relevance to growth and remodeling of the microcirculation remains an open but interesting question, particularly due to its potential implications in arteriogenesis [14,108,123].
In the setting of tumor angiogenesis, bone marrow-derived cells have been shown to differentiate into both pericytes and macrophages [68,207]. The evidence for macrophages and pericytes expressing overlapping sets of surface proteins [143] can make delineating their identity and defining their respective lineages a bit confusing. Additionally, pericytes have a suggested link to fibroblast origins [143], but fibroblasts are not well defined and may represent an ambiguous population of interstitial cells that has overlapping lineage with macrophages and/or pericytes. Macrophage-to-pericyte or macrophage-to-fibroblast transdifferentiation could have important consequences in the regulation of angiogenesis, particularly in settings where high levels of pericyte coverage are essential for maintaining barrier function of the tissue, like in the blood-brain barrier and the blood-retinal barrier, as well as in pathological situations where fibrosis is a contributing factor.
As mentioned above, whether or not endothelial cells (or circulating “endothelial progenitor cells”) arise from the transdifferentiation of monocytes or macrophages has been a topic of great controversy in recent years. Leukocyte sub-populations and circulating progenitor cells have been shown to have overlapping cell surface marker expression profiles [115,225], leading to the suggestion that monocytes/macrophages are capable of incorporating into neovessels as endothelial cells [148]. In 2002, work by Moldovan et al. supported this paradigm by suggesting that macrophages drill interstitial tunnels that can be occupied by endothelial cells, which are themselves derived from macrophages [184]. However, the expression of characteristic endothelial markers (CD31, VEGFR2, and Tie-2) by a subset of monocytes, termed “myeloid angiogenic cells” (MACs) [38], offers a different explanation for the observed phenotypic overlap. While MACs have been shown to be highly angiogenic and resemble alternatively activated M2 macrophages, their inability to differentiate into endothelial cells has been demonstrated in murine models of ischemia [181,280]. The notion of phenotypic overlap between endothelial cells and monocyte-derived macrophages has also been challenged by studies showing that monocytes uptake platelet microparticles bearing endothelial surface markers [218]. While there is now more recent evidence to support the potential for bone marrow myeloid cell differentiation into endothelial progenitor cells, the frequency of this occurrence and the specificity of the eliciting stimuli need to be defined in order to appreciate the relative contribution of this process in different angiogenesis settings. Highly specific genetic reporter-based lineage-tracing studies coupled with dynamic imaging are needed to unequivocally prove or disprove the potential for phenotypic transitions to occur between macrophages and vascular wall cells (i.e. endothelial cells, pericytes, and smooth muscle cells) during angiogenesis.
CONNECTIONS BETWEEN ANGIOGENESIS AND LYMPHANGIOGENESIS
The review of macrophage dynamics during angiogenesis motivates the recognition that macrophages also play similar roles in lymphangiogenesis. Knowing that lymphatic microvascular network coordination with the blood microvasculature plays a key role in normal physiological functions, such as local tissue fluid balance, tissue perfusion, and immune surveillance [56,167,233], and given the importance of lymphangiogenesis during tumor growth and cancer metastasis, it is surprising that more laboratories do not study both angiogenesis and lymphangiogenesis at the same time. Recent work identifying the temporal and spatial relationships between these two processes highlights the importance of investigating both processes at the same time using the same model systems [20,24,272]. Considering the close relationships that macrophages have with blood and lymphatic endothelial cells, studying macrophage behaviors in this context offers a potentially fruitful approach to unite angiogenesis and lymphangiogenesis research.
During embryonic development, Prox1+ endothelial cells bud off of the cardinal vein to form the lymphatic vascular system [299], and in adults it is this Prox1 identity that is required for lymphatic endothelial cell function and identity maintenance. While lymphatic endothelial cells express many of the same markers as blood endothelial cells (von Willebrand factor, anti-thrombin 3, and CD31 [3]), they can be distinguished from blood endothelial cells by lymphatic-specific markers including Prox1, VEGFR3, Podoplanin, and lymphatic endothelial hyaluronan receptor-1 (LYVE-1) [298]. The traditional paradigm that the lymphatic and blood circulations are distinct from one another was first challenged over 30 years ago with the identification of connections between these two systems in the microcirculation of dog hearts after lymphatic obstruction [73]. More recently, research focused on blood/lymphatic vessel mispatterning based on direct lymphatic blood perfusion has supported the existence of direct connections between lymph and blood vessels. Injections of FITC-labeled dextran or BSI-lectin identified blood-to-lymphatic perfusion in mice lacking SLP-76 [1], Rac1 [54], O-glycan [88], or Fiaf [17]. Similar results were documented in Prox1 conditional-mutant mice [132]. There is also support that environmental factors might be sufficient to influence lymphatic identity and mispatterning. Lin et al. demonstrated that LPA causes HUVECs to upregulate lymphatic marker expression in vitro [168]. Cooley et al. showed that HUVECs cultured in three versus two dimensions also are capable of transitioning to a lymphatic identity [48] and Jensen et al., [56] suggested that nitric oxide could induce hypoxia induced lymphatic blood perfusion in zebrafish at sites of arterial-lymphatic anastomoses. So, what we know now is that lymphatic vessels can connect directly to blood vessels and that lymphatic and blood endothelial cells exist as two states along a shared phenotype spectrum[42]. The similarities (rather than differences) between lymphatic and blood endothelial cells offer an opportunity to apply our knowledge about angiogenesis to advance our understanding about lymphangiogenesis, and vice versa. Moreover, the potential link that macrophages may provide -- either physically or by providing paracrine signaling -- between these two circulatory systems should be explored in this context.
As an example, we recently discovered that vascular island incorporation, characterized as the proliferation and connection of disconnected endothelial segments with nearby networks, as a new mode of growth associated with angiogenesis [142,144,263]. Support of this dynamic is provided by the evidence that migration and re-connection of isolated lymphatic endothelial cells segments was already characterized as an accepted mode of lymphangiogenesis [24,141,210,238]. Now questions regarding how vascular islands are guided to connect to either blood or lymphatic networks and, for that matter, how angiogenesis and lymphangiogenesis are interdependently regulated remain to be answered. Interestingly, the observation of lymphatic island formation and fusion to nearby networks during recurrent inflammation-induced lymphangiogenesis correlated with an increase in CD11b positive macrophage accumulation [142]. The possible role of macrophages in what we have termed vascular island incorporation, however, remains to be explored.
We speculate that macrophage dynamics may offer putative mechanistic linkages between angiogenesis and lymphangiogenesis. Just like in angiogenesis, macrophages stimulate lymphangiogenesis via paracrine mechanisms and have been suggested to possess the ability to differentiate into lymphatic endothelial cells [72,102,179]. A future challenge is to determine if macrophages merely are independent players in these vessel growth processes or if they orchestrate coordination between the two.
MACROPHAGE DYNAMICS IN LYMPHANGIOGENESIS
While lymphangiogenesis research is benefiting from its own renaissance, our knowledge still lags what we know about angiogenesis. Compare a recent PubMed search for “macrophages and angiogenesis” resulting in ~3700 articles versus a search for “macrophages and lymphangiogenesis” resulting in ~230. This discrepancy emphasizes the need for more lymphangiogenesis studies focused on the assessment of macrophage dynamics that have been identified during angiogenesis in regulating various aspects of lymphangiogenesis. Snapshots of CD11b+ and LYVE-1+ interstitial cells near and apparently interacting with lymphatic sprouts (Figure 4) suggests that macrophages are involved in lymphangiogenesis in many of the same ways that they are in involved in angiogenesis. In this section, we attempt to synthesize two motivating themes for macrophages dynamics in lymphangiogenesis, paracrine signaling and endothelial cell differentiation, that parallel what have already presented for angiogenesis. For more comprehensive lists of the overlapping roles for macrophages in both processes we suggest the following reviews [3,203,227].
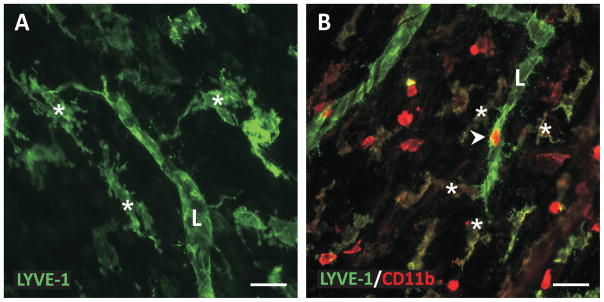
Figure 4. Examples of macrophages nearby lymphatic sprouts during lymphangiogenesis.
A) LYVE-1 positive interstitial cells nearby a LYVE-1 positive lymphatic vessel (L) in cultured rat mesenteric tissues stimulated with VEGF-C. B) LYVE-1/CD11b co-labeling during lymphangiogenesis in the rat mesentery tissue stimulated by chronic inflammation. Cellular extensions from interstitial cells (asterisks) toward the lymphatic vessel support the ability for macrophages to interact with and become lymphatic endothelial cells. Arrowhead identifies a CD11b positive cell along the lymphatic sprout. Scale bars = 50 microns.
Lymphangiogenesis occurs in lymphatic capillaries, referred to as initial lymphatic vessels and consisting of only endothelial cells. In contrast, upstream collecting, or contractile lymphatic vessels, are covered with smooth muscle cells. So, while the roles of macrophages, mast cells, and other immune cell types have been implicated as modulators of smooth muscle cell pumping along collecting lymphatics [40,41,152], macrophage interactions during lymphangiogenesis are limited to the endothelium with their major role attributed to paracrine signaling. Interestingly, observation of pericytes along LYVE-1, Prox1 negative, and Podoplanin-negative endothelial extensions off initial lymphatics implicates a possible transient role for pericytes in some form of lymphangiogenesis [232], yet for now any macrophage-pericyte interaction during this process remains unknown.
Paracrine roles for macrophages in lymphangiogenesis
The main commonality that macrophages exhibit in lymphangiogenesis and angiogenesis is their secretion of paracrine signaling molecules [203,227]. And, as could be expected, macrophages during lymphangiogenesis secrete many of the same molecules that they have been shown to secrete during angiogenesis (VEGF-A, VEGF-C, PDGF, bFGF, TNF-α, MMP-2, MMP-9, Ang2, TGF-β and others (Table 3; [221]), with the most important factor in lymphangiogenesis considered to be VEGF-C. While VEGF-C can also stimulate angiogenesis, it is more often associated with lymphangiogenesis [24,137,260,271]. The recruitment and dramatic accumulation of macrophages and production of VEGF-C during inflammation induced lymphangiogenesis is supported by numerous studies [72,135,138,141,148]. In recognition of its importance, an opportunity exists to link how other molecular signals involved in lymphangiogenesis intersect with VEGF-C. As an example, work by Suzuki et al. suggests that macrophage upregulation of VEGF-C expression leading to lymphangiogenesis is induced by TGF-β [270]. Another example is seen with the tumor nuclear factor-alpha (TNF-α) signaling pathway, which has been reported to depend completely on VEGFR3 activation. Tumor necrosis factor receptor 1 (TNFR1) activation in macrophages by tumor necrosis factor-alpha (TNF-α), one of the prominent pro-inflammatory cytokines, stimulates the secretion of VEGF-C, which in turn activates VEGFR3 on lymphatic endothelial cells [95].
Evidence for macrophage differentiation into lymphatic endothelial cells
In addition to paracrine mechanisms, macrophages have also been suggested contribute to lymphangiogenesis by transdifferentiating into lymphatic endothelial cells [72,102,179]. Most of this evidence is based on the co-labeling of macrophage and lymphatic endothelial cell makers and cell-lineage studies similar to the analogous studies showing macrophage-to-blood endothelial transition [221]. Hall et al., demonstrated via time-lapse analysis of LPS stimulated lymphangiogenesis in the mouse diaphragm that macrophage-derived lymphatic endothelial progenitor cells incorporated into lymphatic vessels and that cultured macrophages can be induced to express lymphatic markers [102]. Similarly, in the study by El-Chemaly et al., Cd11b+ macrophages were shown to form lymphatic like structures in vitro in Matrigel that were positive for LYVE-1 and podoplanin [72]. In an experimental autoimmune orchitis (EAO) model, a model for studying chronic inflammation of the testes, F4/80+ macrophages were found to migrate towards and get incorporated into the lymphatic capillary walls and, intriguingly, not the walls of blood capillaries [111]. In a mouse model of tail lymphedema, Shimizu et al. injected adipose-derived regenerative cells into the skin flap surgically created on the tail and concluded that M2 macrophages could be a source of lymphatic endothelial progenitor cells [255]. Compelling evidence is also provided in work by Maruyama et al., which demonstrates that lymphatic endothelial cells in corneal lymphangiogenesis arise from CD11b+ cells [179].
In light of the convincing evidence for injected lymphatic endothelial progenitor cells to also transdifferentiate into lymphatic endothelial cells and contribute to lymphangiogenesis in various models of injury, inflammation and cancer [47,161,219,226,274,281], critical questions exist regarding how macrophages fit in. Are macrophages a subset of these lymphatic progenitor cell populations or should they be considered a different cell type all together? Lymphatic endothelial cell progenitor cell populations include, for example, adipose derives stem cells, bone marrow derived lymphatic EPCs, CD34+/VEGFR3+ lymphatic/vascular EPCs, and multi-potent mesenchymal stem cells. Similar to the analogous scenario discussed for angiogenesis, future conclusions about macrophage transdifferentiation into vascular wall cells warrant a better understanding of the importance and differences between macrophages and the other progenitor cell types. The relatively low transdifferentiation percentages also raise questions about how much this process contributed to the overall lymphangiogenic effect versus paracrine mechanisms. Regardless, convincing evidence suggests that macrophages can become lymphatic endothelial cells during development and in adult tissues. Additional relevant follow-on questions in the context of lymphangiogenesis and angiogenesis include: do subpopulations of macrophages become lymphatic versus blood endothelial cells, can lymphatic or blood endothelial cells de-differentiate back into macrophages, and how is a macrophage guided to one endothelial cell fate verse the other?
TUMOR-ASSOCIATED MACROPHAGES IN ANGIOGENESIS AND LYMPHANGIOGENESIS AND THERAPEUTIC IMPLICATIONS
Macrophages are known to play important roles in a number of major diseases, including hypertension [174], neurodegenerative disease [29], diabetes [151], and cancer [220]. In the remainder of this section, we will focus on cancer because the roles of macrophages in both tumor angiogenesis and lymphangiogenesis have been a topic of study for quite some time, and recent evidence depicts how influential macrophages can be in these vessel remodeling settings and how macrophages might serve as efficacious therapeutic targets in cancer.
Cancer is an inflammatory process, as the seemingly foreign cells grow and invade healthy tissue, the immune system mounts a response to remove the disease [49]. Due to the ability of cancer cells to adapt to avoid immunity and eventually induce tolerance, this inflammatory process is perpetual. As part of this process, there is aberrant induction of angiogenic and lymphangiogenic processes, which are closely linked to growth and metastasis [36]. However, unlike the vascularization that occurs during development, tumor-associated blood vessels are poorly formed structures with blind ends and large gaps between the endothelial cells, leading to irregular and often increased fluid flux into the tumor. There are numerous outstanding reviews chronicling the structure [79], function [125], mechanistic regulation [296], and inhibition [81,126] of tumor associated blood vessels. Due to an increased interstitial fluid flux into the tumor, along with an increase in solid stress from the tumor cells and ECM, the total interstitial pressure increases 10–100 fold compared to healthy tissue [191]. This change in biophysical forces leads to increased drainage to surrounding healthy tissue and tissue lymphatics. These changes, along with chemical signaling from the tumor microenvironmental cells to the lymphatics, can lead to pathological alterations in the lymph vasculature [261]. Tumor-associated lymphatics (TALs) are not as well-studied as tumor-associated blood vessels, but increasing evidence suggests their importance to cancer progression, particularly metastasis [9]. Lymphangiogenesis in cancer can occur intratumorally (e.g., in melanoma [55], colorectal cancer [8], and inflammatory cancers [188]), peritumorally (e.g., in invasive breast carcinoma [247,248] [67]), or in the draining lymph nodes [155,208]. TAMs are thought to play important roles in both angiogenesis and lymphangiogenesis, through their secretion of pro-angiogenic and pro-lymphangiogenic molecules (VEGF-C, VEGF-D, VEGF-A, TNF-α[45]). It is important to point out that many of the chemokines and growth factors that macrophages secrete, such as VEGF-A and VEGF-C, are also known to elicit mitogenic and chemotactic behaviors in macrophages. Hence, it is important to consider the relative roles of paracrine vs. autocrine signaling within a local tissue environment, and these signaling pathways may be dynamic and spatially heterogeneous – and at this point, difficult to assess in vivo using the tools that are currently available.
Tumor-associated macrophage phenotypes in cancer
TAMs have been implicated in almost every facet of tumor progression to promote malignancy [46]. Macrophages have been implicated in every cancer as an indicator of poor prognosis, except in colorectal where they have been correlated with good prognosis [312]. This apparent contradiction stems from the polarization state of these macrophages [11]. Traditional delineations of classically and alternatively-activated macrophages have been used to identify the subpopulations within cancers. Historically, TAMs have been described as primarily M2-like macrophages, with high expression levels of TGF-β, IL-10, M-CSF, VEGFs, MMPs, TNF-α, and chemokines such as CCL17 and CCL22 [177]. They can be identified primarily by the expression of CD163 and CD68 (which is expressed by all myeloid cells), but can vary in expression of a number of other macrophage differentiation and tissue-specific markers [27]. Recently, the generalization of TAMs as M2-like alternatively activated cells has been challenged with new evidence that a spectrum of macrophages exists in tumors, and the ratio of M2-like/M1 macrophages may in fact be more predictive of poor prognosis in place of total infiltration [136,177]. Current evidence suggests that these populations may vary based on localization within the tumor, with pro-angiogenic M2-like macrophages congregating in areas of hypoxia, and pro-inflammatory M1 macrophages in regions of tissue inflammation [164]. Recent comprehensive reviews highlight the role of TAMs in cancer progression [202] and their therapeutic response [59]. It is important to be cautious when interpreting the existing macrophage classifications in the context of tumors because they are built on emerging evidence, and none of them is universally accepted.
TAMs in tumor angiogenesis
The role of TAMs in the induction of tumor angiogenesis has been observed for decades, both in vitro and in vivo [162]. In patients, a high number of TAMs has been correlated with increased microvessel density in multiple tumor types. Pro-angiogenic Tie2+/CD31+ TAMs produce a number of angiogenic growth factors, namely VEGF-A, bFGF, TGF-α, IL-1β, PDGF-BB, and ECM remodeling molecules, such as the MMPs[59,147,288]. Crossbreeding of the transgenic murine model of breast carcinoma (MMTV-PyMT) with a CSF-1 null mouse results in increased macrophage infiltration, enhanced angiogenesis, and quicker cancer progression[169]. Experiments in Lewis lung carcinoma using clodronate liposomes to deplete macrophages similarly show reductions in both TAMs and angiogenesis[317]. Thus, there is a direct link between macrophages and tumor angiogenesis that is likely due to TAM-mediated modulation of the local growth factor environment and/or, perhaps, by differentiating into tumor endothelial cells.
TAMs in tumor lymphangiogenesis
More recently, the role of TAMs in lymphangiogenesis has been explored in the context of the tumor microenvironment. TAMs have been implicated in encouraging tumor cell migration towards lymphatics and lymph nodes, modulating the inflammatory microenvironment to induce lymphangiogenesis, and priming tumor draining lymph nodes. VEGF-C-expressing TAMs correlate with high lymphatic vessel density in pancreatic carcinoma [155], gastric cancer [8], skin carcinoma, [188] and lymph node positive breast carcinoma [248]. Artificially increasing VEGF-A and VEGF-C in fibrosarcoma cells implanted into the avascular mouse cornea showed increases in inflammatory macrophages in addition to increases in intratumoral lymphangiogenesis (for VEGF-C) and peritumoral lymphangiogenesis (for VEGF-A) [28]. Soluble VEGFR3 delivered to orthotopic models of bladder cancer reduce lymphangiogenesis and metastasis. These effects were primarily mediated by macrophages which were identified as the primary contributors to VEGFC in the tumors [302].
In vitro, macrophages migrate towards VEGF-A and VEGF-C, and in vivo, tumors with high levels of these growth factors have increased macrophage infiltration. However, macrophages also secrete VEGF-A and VEGF-C, increasing the angiogenic microenvironment prior to lymphangiogenesis [227]. Recently Cao and colleagues showed that TAMs secrete VEGF-C in response to TNF-α activation, acting to replace previously reported direct angiogenic and lymphangiogenic effects of TNF-α in Lewis lung carcinoma and ovarian cancer models [131]. In a number of clinical cohorts and murine models of cancers, subsets of TAMs have also been shown to express lymphatic endothelial cell markers, including the VEGF-C receptor, VEGFR3 [256], LYVE1 [245]. VEGFR3+ macrophages are found in multiple types of cancers, including inflammatory skin and breast cancers [10,256], and Lyve1+/CD11b+ macrophages associate with aberrant lymphatics throughout the mesentery in murine models of ovarian cancer [130]. It has been suggested that these particular populations of macropahges may serve as lymphatic endothelial progenitor cells (for a recent review see [221]). Coukos and colleagues harvested bone-marrow derived cells from patients and differentiated these towards a lymphatic endothelial progenitor phenotype which was CD11b+/C14+/VEGFR3+/GP38+/Lyve1+[281]. These cells could be differentiated into lymphatic endothelial cells and secrete VEGFC when exposed to breast cancer patient-derived serum. Interestingly, these populations of lymphatic vascular endothelial progenitor cells can be found circulating in the bloodstream of patients with lung cancer and elevated populations correlate with poor prognosis [30]. These populations of progenitors are just being elucidated in the context of cancer for their role in both angiogenesis and lymphangiogenesis. It is interesting to speculate how the parallel roles that TAMs play in angiogenesis and lymphangiogenesis – in secreting pro-angiogenic and pro-lymphangiogenic growth factors and in potentially serving as a source of endothelial progenitor cells – might serve to link these two vessel growth and remodeling processes and offer a therapeutic target with a double-edged sword.
Targeting TAMs to treat cancer
The landscape of cancer therapy is becoming more complex as new immunotherapies are introduced and chemotherapy regimens become personalized. Evidence suggests that the tumor vasculature [126] and TAMs [59] are involved in the success or failure of most therapeutic strategies and may offer intriguing targets for adjuvant treatment. Therefore, pharmacological blockade of TAMs, angiogenesis, and lymphangiogenesis are ongoing approaches for cancer therapy. Targeting angiogenesis via VEGFR2 blocking antibodies, such as bevacizumab, in murine models of pancreatic cancer reduces the population of intratumoral TAMs [66]. Similarly, inhibition of VEGFR3 with a small molecule inhibitor in the 4T1 murine model of breast carcinoma and Rip1-Tag2 model of pancreatic cancer reduces number of infiltrating TAMs [7]. Conversely, TAM inhibition via CSF1R knockout leads to alterations in angiogenesis in lymphangiogenesis in multiple cancer models [153]. Pharmacological inhibition using anti-M-CSF1R therapeutic antibodies decreases angiogenesis and lymphangiogenesis [230] and can enhance the benefit of immunotherapy in murine models of pancreatic cancer [315]. Inhibition of TAMs leads to an overall reduction in growth and metastasis in cancers, and thus is a promising strategy for therapy alone or in conjunction with traditional approaches.
Interestingly, current standard of care therapies have been tied to increased angiogenesis, lymphangiogenesis and macrophage infiltration. Radiation therapy, common in most cancers, induces infiltration of VEGFC+ CD68+ macrophages and blood vessel expansion 2–8 weeks post therapy but increased lymphatic vessel density one year after therapy [124]. Paclitaxel, another common therapy, induces intratumoral lymphangiogenesis in murine models of breast cancer, potentially through recruitment of myeloid cells, leading to chemoresistant tumors [289]. Alteration of the activation state of TAMs towards an immunogenic phenotype augments the benefit of immunotherapy in murine models of lung cancer [87]. These complex therapeutic interactions, coupled with the complex interactions of macrophages with blood vessels, lymphatic vessels, and other stromal cells in the tumor environment, create unique therapeutic opportunities that lie at the union of tumor angiogenesis and lymphangiogenesis.
SUMMARY AND FUTURE PERSPECTIVES
From development to adult tissue regeneration and disease scenarios, the macrophage is an inflammatory cell “integrator” that links different areas of vascular biology, including angiogenesis and lymphangiogenesis. While their origin, identity, and sub-type classifications are active areas of study in the field of immunology, macrophage presence and involvement in multiple facets of angiogenesis and lymphangiogenesis are becoming studied more widely by the vascular biology community. A better understanding of macrophages and their interactions with endothelial cells, stem cells, pericytes, and other interstitial cells may inspire new therapeutic strategies for regenerating tissues and curing diseases where deregulated angiogenesis and lymphangiogenesis are culprits, such as in cancer.
While this review is an attempt to highlight the current state of knowledge with regards to macrophage dynamics in angiogenesis and their overlapping roles in lymphangiogenesis, more questions remain unanswered than answered. For example, we focused on macrophage involvement in blood and lymphatic capillary sprouting. Almost nothing remains known about their involvement in other modes of microvessel growth, such as intussusception or vascular island incorporation. The extent to which developmental programs regarding macrophage dynamics are reinstituted during adult microvessel remodeling is an also open question, and the genetic and epigenetic regulatory controls that govern macrophage phenotype (and phenotype switching) in remodeling tissues are not well defined. There may exist opportunities to advance our understanding of interactions between macrophages and lymphatic endothelial cells based on our knowledge of how macrophages interact with blood endothelial cells. Last but not least, the relative contribution of macrophages to angiogenesis and lymphangiogenesis compared to other cell types, and their suitability for therapeutic targeting are only beginning to be evaluated [16].
Recognition of the importance of macrophages in microvessel wall growth is not new [269]; however, macrophages are re-emerging as a cell type of interest in angiogenesis and lymphangiogenesis. As the spotlight turns to macrophages in these dynamic processes, there is a growing appreciation that we cannot necessarily rely on single time-point “snapshots” of macrophages in tissues that use phenotypic markers to delineate them from other cell types. Because of their spectrum of dynamic behaviors, we submit there is an opportunity to apply in vivo lineage tracing and depletion models to gain further insights (Table 4). Furthermore, there have been significant advances in targeted drug delivery to allow researchers to pharmacologically manipulate certain pathways within macrophages, using agents such as liposomes conjugated with macrophage targeting antibodies or ligands (anti-VCAM-1 and CD169 ligand), lectins (mannosylated, oligmannosylated), or peptides (muramyl tripeptides) [139,145]. Alternatively, new delivery systems such as microspheres, nanovesicles, and niosomes can target macrophages in the absence of additional active targeting moieties [154,291]. These tools, when used in combination with standard in vivo assays of angiogenesis and lymphangiogenesis, may prove to answer many of the provocative questions that we have put forth throughout this review. Finally, we submit that the development and deployment of new computational modeling approaches [19,211] will enable in silico hypothesis testing, provide unparalleled quantitative descriptions of the interactions between macrophages and vascular wall cells that may be important in regulating angiogenesis and lymphangiogenesis, and facilitate the design of “smart” therapies that account for and/or harness macrophage dynamics to control microvessel growth.
Table 4.
Small animal models that have been used to study monocyte/macrophage trafficking and function
| In Vivo Macrophage Tracking Models in Mice | |||
|---|---|---|---|
| Name | Cell Typesa | Considerations | Reference |
| CD68-GFP1 | All Mφ | Cannot distinguish Mφ subpopulations. Not known if perivascular Mφ included. | [121] |
| CX3CR1-GFP2 | Monocytes, RMφ, NK, DC, Microglia. | Degraded Mφ chemotaxis in homozygous knock-in, alters macrophage behavior. Minor effect with heterozygous. | [134] |
| CCR2-RFP3 | Monocytes, RMφ, NK, T Cells | Can track recently extravasated macrophages: CCR2 expression is lost over time. Heterozygous mouse has reduced migration from BM and extravasation, severe for homozygous. | [240,284,311] |
| DPE-GFP | PVMφ, αβ-T Cells | Only demonstrated in dermal tissue, not known if constitutive, nor proportion of PVMφ population included. | [2] |
| CSF1R-EGFP | TRMφ, All myeloid cells. | No ability to trace lineage in inflammation. Although used to study TRMφ, many cell types marked according to other sources. | [120,185,242] |
| CX3CR1CreER: R26-YFP | Monocytes, RMφ, TRMφ | TMX induction can be tuned for lineage tracing of different cells. Adult cell types with particular TMX induction protocol must be validated. | [94,305] |
| Gamma Irradiation and GFP BMT | All adult BM-derived cells | Extensively used model. Non-physiologic whole body injury increases RMφ contribution to tissues, partial depletion of TRMφ populations. | [292] |
| Csf1rCre/WT: Rosa26eYFP | Mφ derived from YS-Mφ, TRMφ | Single TMX injection leads to incomplete induction. | [114] |
| S100a4Cre/WT: Rosa26eYFP | Mφ derived from fetal liver monocytes, TRMφ | Single TMX injection leads to incomplete induction. | [114] |
| Runx1Cre/WT: Rosa26eYFP | Fetal lymphoid progenitors and adult HSCs | Single TMX injection leads to incomplete induction. Unclear which cells in adult included. | [241] |
| CAG-GFP Parabiosis with γ Irradiation | All myeloid lineage except erythrocytes. | Avoid bone marrow transplantation to track myeloid cells. Irradiation causes whole body injury, non-physiologic conditions. | [6] |
| BM Transplant with Busulfan | All myeloid lineage | Bone marrow chimera that avoids irradiation and whole body injury, but tracks a multitude of cells types. | [146] |
| GFP Parabosis | All myeloid lineage | Labels minority of cell populations (30%). | [5,105] |
| Models of Macrophage Depletion | |||
|---|---|---|---|
| Name | Depleted Cell Typesa | Considerations | Reference |
| CD11b-DTR | Monocytes, Mφ, Neutrophils | Effective acute depletion of target cells, possible to acquire immunity to DT treatments, produces low grade inflammation, multiple cell types [43] | [71,194] |
| CD169-DTR | RMφ, TRMφ | Possible to acquire immunity to DT treatments, produces low grade inflammation [43] | [44] |
| CD68-DTA | All Mφ | Only 50% depletion of CD68+ cells. | [92] |
| CD11b-HSVTK | Monocytes, Mφ, Neutrophils | Unknown efficacy for TRMφ. Can produce low grade inflammation from apoptosis [43]. | [109] |
| Chlodronate Liposomes | Monocyte, Mφ | Can deplete TRMφ or Monocytes/Mφ depending on method of administration. Any leakage of compound from cells can directly inhibit angiogenesis [228]. Low grade inflammation from apoptotic cells [43]. | [285,286] |
| LysM-Cre/DTR | All myeloid lineage | Constitutive cre expression of DTR improves ablation efficacy. Depletion of multiple cell types. | [97] |
| LysMCrex CSF1RLsL-DTR |
Monocyte, Mφ | Elegant genetic approach to target cell populations. Inconsistent TRMφ depletion between tissues, perhaps only monocyte-derived subgroups. | [249] |
| Neutralizing CSF1R Antibody | TRMφ | Targets tissue resident macrophages. Variable and incomplete ablation efficacy (~80%) in peripheral tissue, not fully validated. | [173] |
Abbreviations: Mφ: macrophages, RMφ: recruited macrophages, TRMφ: tissue resident macrophages, PVMφ: perivascular macrophages, YS-Mφ: yolk sack macrophages, TMX: tamoxifen. GFP: green fluorescent protein, EGFP: enhanced green fluorescent protein, RFP: red fluorescent protein, YFP: yellow fluorescent protein, CRE: cre recombinase, CRE-ER: cre recombinase fused with human estrogen receptor 2, WT: wild type. CD68: cluster of differentiation 68, CX3CR1: chemokine (C-X3-C motif) receptor 1, CCR2: chemokine (C-C motif) receptor 2, DPE, CSF1R: Colony Stimulating Factor 1 Receptor, BMT: bone marrow transplant, S100a4: S100 Calcium Binding Protein A4, Runx1: Runt-Related Transcription Factor 1, CAG: CMV early enhancer/chicken beta actin, DTR: diptheria toxin receptor. DTA: diptheria toxin fragment A. HSVTK: herpes simplex virus thymidine kinase type 1 gene, CD11b: Integrin, Alpha M (Complement Component 3 Receptor 3 Subunit), CD169: Sialic Acid Binding Ig-Like Lectin 1, LysM: Putative Peptidoglycan-Binding, Domain Containing 4.
Acknowledgments
This work was supported by the Tulane Center for Aging and NIH 5-P20GM103629-04 to W.L.M.; NIH EY022063, NIH HL082838, and The Hartwell Foundation to S.M.P.; NIH T32GM008715 to B.A.C.; and IRG 81-001-26 from the American Cancer Society to J.M. The authors would also like to thank Scott Seaman, Anthony Bruce, and Shannan Petchul for their contributions of micrograph panels to Figure 3 and for their assistance in editing the manuscript and providing reference suggestions for the text.
ABBREVIATIONS
- Ang-2
angiopoietin-2
- BSI
Bandeiraea simplicifolia (Griffonia simplicifolia)
- CCL2/MCP-1
chemokine (C-C motif) ligand 2
- CCL17
chemokine (C-C motif) ligand 17
- CCL22
chemokine (C-C motif) ligand 22
- CCR2
chemokine (C-C motif) receptor type 2
- CD11b
cluster of differentiation 11b
- CD163
cluster of differentiation 163
- CD206
cluster of differentiation 206
- CD31
cluster of differentiation 31
- CD68
cluster of differentiation 68
- CSF1
colony stimulating factor 1
- CSF1R
colony stimulating factor 1 receptor
- CX3CR1
chemokine (C-X3-C motif) receptor 1
- ECM
extracellular matrix
- EMPs
erythro-myeloid progenitors
- EPC
endothelial progenitor cell
- FACS
fluorescence activated cell sorting
- bFGF
basic fibroblast growth factor
- FGF2
basic fibroblast growth factor 2
- F4/80
EGF-like module-containing mucin-like hormone receptor-like 1
- Fiaf
fasting-induced adipose factor
- FISH
fluorescent in situ hybridization
- FITC
fluorescein isothiocyanate
- GFP
green fluorescent protein
- GM-CSF
granulocyte macrophage colony-stimulating factor
- HPMCs
human peritoneal mesothelial cells
- HUVEC
human umbilical vein endothelial cells
- ICAM-1
intercellular adhesion molecule-1
- IFN-γ
interferon gamma
- iNOS
inducible nitric oxide synthase
- IL-1
interleukin-1
- IL-4
interleukin-4
- IL-6
interleukin-6
- IL-10
interleukin-10
- IL-12
interleukin-12
- IL-23
interleukin-23
- Klf4
krüppel-like factor 4
- LPA
lysophosphatidic acid
- LPS
lipopolysaccharide
- Ly6C
lymphocyte antigen 6 complex
- LYVE-1
lymphatic endothelial hyaluronan receptor-1
- MAC
myeloid angiogenic cells
- Mif
macrophage migration inhibitory factor
- M-CSF
macrophage colony-stimulating factor
- MMP-2
matrix metaolprotinase-2
- MMP-9
matrix metaolprotinase-9
- MMTV
mouse mammary tumor virus promoter
- PDGF
platelet derived growth factor
- PDGFβ
platelet derived growth factor beta
- Prox1
Prospero Homeobox 1
- PyMT
polyoma virus middle T antigen transgene
- Rac1
RAS-related C3 botulinum substrate 1
- Rip1
rat insulin promoter 1
- SIX1
sine oculis homeobox homeobox 1
- SLP-76
lymphocyte cytosolic protein 2
- SMC
smooth muscle cell
- Tag2
SV40 large t antigen transgene
- TAL
tumor-associated lymphatic
- TAM
tumor-associated macrophage
- Tie-2
receptor tyrosine kinase of the Tie family
- TGF-β
transforming growth factor-beta
- TNF-α
tumor necrosis factor-alpha
- TNFR1
Tumor necrosis factor receptor 1
- VEGF
vascular endothelial growth factor family
- VEGFR2
vascular endothelial growth factor receptor-2
- VEGFR3
vascular endothelial growth factor receptor-3
References
- 1.Abtahian F, Guerriero A, Sebzda E, Lu MM, Zhou R, Mocsai A, Myers EE, Huang B, Jackson DG, Ferrari VA, Tybulewicz V, Lowell CA, Lepore JJ, Koretzky GA, Kahn ML. Regulation of blood and lymphatic vascular separation by signaling proteins SLP-76 and Syk. Science (New York, NY ) 2003;299:247–251. doi: 10.1126/science.1079477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abtin A, Jain R, Mitchell AJ, Roediger B, Brzoska AJ, Tikoo S, Cheng Q, Ng LG, Cavanagh LL, von Andrian UH, Hickey MJ, Firth N, Weninger W. Perivascular macrophages mediate neutrophil recruitment during bacterial skin infection. Nat Immunol. 2014;15:45–53. doi: 10.1038/ni.2769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adams RH, Alitalo K. Molecular regulation of angiogenesis and lymphangiogenesis. Nature reviews. Molecular cell biology. 2007;8:464–478. doi: 10.1038/nrm2183. [DOI] [PubMed] [Google Scholar]
- 4.Aichele P, Zinke J, Grode L, Schwendener RA, Kaufmann SHE, Seiler P. Macrophages of the Splenic Marginal Zone Are Essential for Trapping of Blood-Borne Particulate Antigen but Dispensable for Induction of Specific T Cell Responses. The Journal of Immunology. 2003;171:1148–1155. doi: 10.4049/jimmunol.171.3.1148. [DOI] [PubMed] [Google Scholar]
- 5.Ajami B, Bennett JL, Krieger C, McNagny KM, Rossi FM. Infiltrating monocytes trigger EAE progression, but do not contribute to the resident microglia pool. Nat Neurosci. 2011;14:1142–1149. doi: 10.1038/nn.2887. [DOI] [PubMed] [Google Scholar]
- 6.Ajami B, Bennett JL, Krieger C, Tetzlaff W, Rossi FM. Local self-renewal can sustain CNS microglia maintenance and function throughout adult life. Nat Neurosci. 2007;10:1538–1543. doi: 10.1038/nn2014. [DOI] [PubMed] [Google Scholar]
- 7.Alam A, Blanc I, Gueguen-Dorbes G, Duclos O, Bonnin J, Barron P, Laplace MC, Morin G, Gaujarengues F, Dol F, Herault JP, Schaeffer P, Savi P, Bono F. SAR131675, a potent and selective VEGFR-3-TK inhibitor with antilymphangiogenic, antitumoral, and antimetastatic activities. Mol Cancer Ther. 2012;11:1637–1649. doi: 10.1158/1535-7163.MCT-11-0866-T. [DOI] [PubMed] [Google Scholar]
- 8.Algars A, Irjala H, Vaittinen S, Huhtinen H, Sundstrom J, Salmi M, Ristamaki R, Jalkanen S. Type and location of tumor-infiltrating macrophages and lymphatic vessels predict survival of colorectal cancer patients. Int J Cancer. 2012;131:864–873. doi: 10.1002/ijc.26457. [DOI] [PubMed] [Google Scholar]
- 9.Alitalo A, Detmar M. Interaction of tumor cells and lymphatic vessels in cancer progression. Oncogene. 2012;31:4499–4508. doi: 10.1038/onc.2011.602. [DOI] [PubMed] [Google Scholar]
- 10.Alitalo AK, Proulx ST, Karaman S, Aebischer D, Martino S, Jost M, Schneider N, Bry M, Detmar M. VEGF-C and VEGF-D blockade inhibits inflammatory skin carcinogenesis. Cancer Res. 2013;73:4212–4221. doi: 10.1158/0008-5472.CAN-12-4539. [DOI] [PubMed] [Google Scholar]
- 11.Allavena P, Sica A, Garlanda C, Mantovani A. The Yin-Yang of tumor-associated macrophages in neoplastic progression and immune surveillance. Immunological reviews. 2008;222:155–161. doi: 10.1111/j.1600-065X.2008.00607.x. [DOI] [PubMed] [Google Scholar]
- 12.Allavena P, Sica A, Solinas G, Porta C, Mantovani A. The inflammatory micro-environment in tumor progression: the role of tumor-associated macrophages. Crit Rev Oncol Hematol. 2008;66:1–9. doi: 10.1016/j.critrevonc.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 13.Amos PJ, Kapur SK, Stapor PC, Shang H, Bekiranov S, Khurgel M, Rodeheaver GT, Peirce SM, Katz AJ. Human adipose-derived stromal cells accelerate diabetic wound healing: impact of cell formulation and delivery. Tissue engineering. Part A. 2010;16:1595–1606. doi: 10.1089/ten.tea.2009.0616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arras M, Ito WD, Scholz D, Winkler B, Schaper J, Schaper W. Monocyte activation in angiogenesis and collateral growth in the rabbit hindlimb. J Clin Invest. 1998;101:40–50. doi: 10.1172/JCI119877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science (New York, NY ) 1997;275:964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 16.Awojoodu AO, Ogle ME, Sefcik LS, Bowers DT, Martin K, Brayman KL, Lynch KR, Peirce-Cottler SM, Botchwey E. Sphingosine 1-phosphate receptor 3 regulates recruitment of anti-inflammatory monocytes to microvessels during implant arteriogenesis. Proc Natl Acad Sci U S A. 2013;110:13785–13790. doi: 10.1073/pnas.1221309110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Backhed F, Crawford PA, O’Donnell D, Gordon JI. Postnatal lymphatic partitioning from the blood vasculature in the small intestine requires fasting-induced adipose factor. Proc Natl Acad Sci U S A. 2007;104:606–611. doi: 10.1073/pnas.0605957104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bailey AM, Lawrence MB, Shang H, Katz AJ, Peirce SM. Agent-based model of therapeutic adipose-derived stromal cell trafficking during ischemia predicts ability to roll on P-selectin. PLoS Comput Biol. 2009;5:e1000294. doi: 10.1371/journal.pcbi.1000294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bailey AM, Thorne BC, Peirce SM. Multi-cell agent-based simulation of the microvasculature to study the dynamics of circulating inflammatory cell trafficking. Annals of biomedical engineering. 2007;35:916–936. doi: 10.1007/s10439-007-9266-1. [DOI] [PubMed] [Google Scholar]
- 20.Baluk P, Tammela T, Ator E, Lyubynska N, Achen MG, Hicklin DJ, Jeltsch M, Petrova TV, Pytowski B, Stacker SA, Yla-Herttuala S, Jackson DG, Alitalo K, McDonald DM. Pathogenesis of persistent lymphatic vessel hyperplasia in chronic airway inflammation. J Clin Invest. 2005;115:247–257. doi: 10.1172/JCI22037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bartneck M, Heffels KH, Pan Y, Bovi M, Zwadlo-Klarwasser G, Groll J. Inducing healing-like human primary macrophage phenotypes by 3D hydrogel coated nanofibres. Biomaterials. 2012;33:4136–4146. doi: 10.1016/j.biomaterials.2012.02.050. [DOI] [PubMed] [Google Scholar]
- 22.Becher B, Schlitzer A, Chen J, Mair F, Sumatoh HR, Teng KW, Low D, Ruedl C, Riccardi-Castagnoli P, Poidinger M, Greter M, Ginhoux F, Newell EW. High-dimensional analysis of the murine myeloid cell system. Nat Immunol. 2014;15:1181–1189. doi: 10.1038/ni.3006. [DOI] [PubMed] [Google Scholar]
- 23.Bechmann I, Kwidzinski E, Kovac AD, Simburger E, Horvath T, Gimsa U, Dirnagl U, Priller J, Nitsch R. Turnover of rat brain perivascular cells. Exp Neurol. 2001;168:242–249. doi: 10.1006/exnr.2000.7618. [DOI] [PubMed] [Google Scholar]
- 24.Benest AV, Harper SJ, Herttuala SY, Alitalo K, Bates DO. VEGF-C induced angiogenesis preferentially occurs at a distance from lymphangiogenesis. Cardiovasc Res. 2008;78:315–323. doi: 10.1093/cvr/cvm094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bergers G, Brekken R, McMahon G, Vu TH, Itoh T, Tamaki K, Tanzawa K, Thorpe P, Itohara S, Werb Z, Hanahan D. Matrix metalloproteinase-9 triggers the angiogenic switch during carcinogenesis. Nat Cell Biol. 2000;2:737–744. doi: 10.1038/35036374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Besner GE, Klagsbrun M. Macrophages secrete a heparin-binding inhibitor of endothelial cell growth. Microvasc Res. 1991;42:187–197. doi: 10.1016/0026-2862(91)90086-q. [DOI] [PubMed] [Google Scholar]
- 27.Biswas SK, Mantovani A. Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nat Immunol. 2010;11:889–896. doi: 10.1038/ni.1937. [DOI] [PubMed] [Google Scholar]
- 28.Bjorndahl M, Cao R, Nissen LJ, Clasper S, Johnson LA, Xue Y, Zhou Z, Jackson D, Hansen AJ, Cao Y. Insulin-like growth factors 1 and 2 induce lymphangiogenesis in vivo. Proc Natl Acad Sci U S A. 2005;102:15593–15598. doi: 10.1073/pnas.0507865102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bogie JF, Stinissen P, Hendriks JJ. Macrophage subsets and microglia in multiple sclerosis. Acta neuropathologica. 2014;128:191–213. doi: 10.1007/s00401-014-1310-2. [DOI] [PubMed] [Google Scholar]
- 30.Bogos K, Renyi-Vamos F, Dobos J, Kenessey I, Tovari J, Timar J, Strausz J, Ostoros G, Klepetko W, Ankersmit HJ, Lang G, Hoda MA, Nierlich P, Dome B. High VEGFR-3-positive circulating lymphatic/vascular endothelial progenitor cell level is associated with poor prognosis in human small cell lung cancer. Clinical cancer research: an official journal of the American Association for Cancer Research. 2009;15:1741–1746. doi: 10.1158/1078-0432.CCR-08-1372. [DOI] [PubMed] [Google Scholar]
- 31.Brown KJ, Maynes SF, Bezos A, Maguire DJ, Ford MD, Parish CR. A novel in vitro assay for human angiogenesis. Lab Invest. 1996;75:539–555. [PubMed] [Google Scholar]
- 32.Bruce AC, Kelly-Goss MR, Heuslein JL, Meisner JK, Price RJ, Peirce SM. Monocytes are recruited from venules during arteriogenesis in the murine spinotrapezius ligation model. Arterioscler Thromb Vasc Biol. 2014;34:2012–2022. doi: 10.1161/ATVBAHA.114.303399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bruce AC, Peirce SM. Exogenous thrombin delivery promotes collateral capillary arterialization and tissue reperfusion in the murine spinotrapezius muscle ischemia model. Microcirculation (New York, NY : 1994) 2012;19:143–154. doi: 10.1111/j.1549-8719.2011.00138.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bryant-Hudson KM, Gurung HR, Zheng M, Carr DJ. Tumor necrosis factor alpha and interleukin-6 facilitate corneal lymphangiogenesis in response to herpes simplex virus 1 infection. J Virol. 2014;88:14451–14457. doi: 10.1128/JVI.01841-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cao R, Bjorndahl MA, Religa P, Clasper S, Garvin S, Galter D, Meister B, Ikomi F, Tritsaris K, Dissing S, Ohhashi T, Jackson DG, Cao Y. PDGF-BB induces intratumoral lymphangiogenesis and promotes lymphatic metastasis. Cancer Cell. 2004;6:333–345. doi: 10.1016/j.ccr.2004.08.034. [DOI] [PubMed] [Google Scholar]
- 36.Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407:249–257. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- 37.Cassado Ados A, D’Imperio Lima MR, Bortoluci KR. Revisiting mouse peritoneal macrophages: heterogeneity, development, and function. Front Immunol. 2015;6:225. doi: 10.3389/fimmu.2015.00225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chambers SE, O’Neill CL, O’Doherty TM, Medina RJ, Stitt AW. The role of immune-related myeloid cells in angiogenesis. Immunobiology. 2013;218:1370–1375. doi: 10.1016/j.imbio.2013.06.010. [DOI] [PubMed] [Google Scholar]
- 39.Chappell JC, Song J, Klibanov AL, Price RJ. Ultrasonic microbubble destruction stimulates therapeutic arteriogenesis via the CD18-dependent recruitment of bone marrow-derived cells. Arterioscler Thromb Vasc Biol. 2008;28:1117–1122. doi: 10.1161/ATVBAHA.108.165589. [DOI] [PubMed] [Google Scholar]
- 40.Chatterjee V, Gashev AA. Aging-associated shifts in functional status of mast cells located by adult and aged mesenteric lymphatic vessels. American journal of physiology. Heart and circulatory physiology. 2012;303:H693–702. doi: 10.1152/ajpheart.00378.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chatterjee V, Gashev AA. Mast cell-directed recruitment of MHC class II positive cells and eosinophils towards mesenteric lymphatic vessels in adulthood and elderly. Lymphatic research and biology. 2014;12:37–47. doi: 10.1089/lrb.2013.0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen CY, Bertozzi C, Zou Z, Yuan L, Lee JS, Lu M, Stachelek SJ, Srinivasan S, Guo L, Vicente A, Mericko P, Levy RJ, Makinen T, Oliver G, Kahn ML. Blood flow reprograms lymphatic vessels to blood vessels. J Clin Invest. 2012;122:2006–2017. doi: 10.1172/JCI57513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chow A, Brown BD, Merad M. Studying the mononuclear phagocyte system in the molecular age. Nat Rev Immunol. 2011;11:788–798. doi: 10.1038/nri3087. [DOI] [PubMed] [Google Scholar]
- 44.Chow A, Huggins M, Ahmed J, Hashimoto D, Lucas D, Kunisaki Y, Pinho S, Leboeuf M, Noizat C, van Rooijen N, Tanaka M, Zhao ZJ, Bergman A, Merad M, Frenette PS. CD169(+) macrophages provide a niche promoting erythropoiesis under homeostasis and stress. Nat Med. 2013;19:429–436. doi: 10.1038/nm.3057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Coffelt SB, Hughes R, Lewis CE. Tumor-associated macrophages: effectors of angiogenesis and tumor progression. Biochimica et biophysica acta. 2009;1796:11–18. doi: 10.1016/j.bbcan.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 46.Condeelis J, Pollard JW. Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell. 2006;124:263–266. doi: 10.1016/j.cell.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 47.Conrad C, Niess H, Huss R, Huber S, von Luettichau I, Nelson PJ, Ott HC, Jauch KW, Bruns CJ. Multipotent mesenchymal stem cells acquire a lymphendothelial phenotype and enhance lymphatic regeneration in vivo. Circulation. 2009;119:281–289. doi: 10.1161/CIRCULATIONAHA.108.793208. [DOI] [PubMed] [Google Scholar]
- 48.Cooley LS, Handsley MM, Zhou Z, Lafleur MA, Pennington CJ, Thompson EW, Poschl E, Edwards DR. Reversible transdifferentiation of blood vascular endothelial cells to a lymphatic-like phenotype in vitro. J Cell Sci. 2010;123:3808–3816. doi: 10.1242/jcs.064279. [DOI] [PubMed] [Google Scholar]
- 49.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cronk SM, Kelly-Goss MR, Ray HC, Mendel TA, Hoehn KL, Bruce AC, Dey BK, Guendel AM, Tavakol DN, Herman IM, Peirce SM, Yates PA. Adipose-derived stem cells from diabetic mice show impaired vascular stabilization in a murine model of diabetic retinopathy. Stem cells translational medicine. 2015;4:459–467. doi: 10.5966/sctm.2014-0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cunningham C. Microglia and neurodegeneration: the role of systemic inflammation. Glia. 2013;61:71–90. doi: 10.1002/glia.22350. [DOI] [PubMed] [Google Scholar]
- 52.Cursiefen C, Chen L, Borges LP, Jackson D, Cao J, Radziejewski C, D’Amore PA, Dana MR, Wiegand SJ, Streilein JW. VEGF-A stimulates lymphangiogenesis and hemangiogenesis in inflammatory neovascularization via macrophage recruitment. Journal of Clinical Investigation. 2004;113:1040–1050. doi: 10.1172/JCI20465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cursiefen C, Maruyama K, Bock F, Saban D, Sadrai Z, Lawler J, Dana R, Masli S. Thrombospondin 1 inhibits inflammatory lymphangiogenesis by CD36 ligation on monocytes. J Exp Med. 2011;208:1083–1092. doi: 10.1084/jem.20092277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.D’Amico G, Jones DT, Nye E, Sapienza K, Ramjuan AR, Reynolds LE, Robinson SD, Kostourou V, Martinez D, Aubyn D, Grose R, Thomas GJ, Spencer-Dene B, Zicha D, Davies D, Tybulewicz V, Hodivala-Dilke KM. Regulation of lymphatic-blood vessel separation by endothelial Rac1. Development. 2009;136:4043–4053. doi: 10.1242/dev.035014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dadras SS, Paul T, Bertoncini J, Brown LF, Muzikansky A, Jackson DG, Ellwanger U, Garbe C, Mihm MC, Detmar M. Tumor lymphangiogenesis: a novel prognostic indicator for cutaneous melanoma metastasis and survival. Am J Pathol. 2003;162:1951–1960. doi: 10.1016/S0002-9440(10)64328-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dahl Ejby Jensen L, Cao R, Hedlund EM, Soll I, Lundberg JO, Hauptmann G, Steffensen JF, Cao Y. Nitric oxide permits hypoxia-induced lymphatic perfusion by controlling arterial-lymphatic conduits in zebrafish and glass catfish. Proc Natl Acad Sci U S A. 2009;106:18408–18413. doi: 10.1073/pnas.0907608106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Davies LC, Jenkins SJ, Allen JE, Taylor PR. Tissue-resident macrophages. Nat Immunol. 2013;14:986–995. [Google Scholar]
- 58.Davies LC, Jenkins SJ, Allen JE, Taylor PR. Tissue-resident macrophages. Nat Immunol. 2013;14:986–995. [Google Scholar]
- 59.De Palma M, Lewis CE. Macrophage regulation of tumor responses to anticancer therapies. Cancer Cell. 2013;23:277–286. doi: 10.1016/j.ccr.2013.02.013. [DOI] [PubMed] [Google Scholar]
- 60.De Palma M, Venneri MA, Galli R, Sergi Sergi L, Politi LS, Sampaolesi M, Naldini L. Tie2 identifies a hematopoietic lineage of proangiogenic monocytes required for tumor vessel formation and a mesenchymal population of pericyte progenitors. Cancer Cell. 2005;8:211–226. doi: 10.1016/j.ccr.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 61.DeFalco T, Bhattacharya I, Williams AV, Sams DM, Capel B. Yolk-sac-derived macrophages regulate fetal testis vascularization and morphogenesis. Proc Natl Acad Sci U S A. 2014;111:E2384–2393. doi: 10.1073/pnas.1400057111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Deryugina EI, Quigley JP. Tumor angiogenesis: MMP-mediated induction of intravasation- and metastasis-sustaining neovasculature. Matrix biology: journal of the International Society for Matrix Biology. 2015;44–46C:94–112. doi: 10.1016/j.matbio.2015.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Deshmane SL, Kremlev S, Amini S, Sawaya BE. Monocyte chemoattractant protein-1 (MCP-1): an overview. J Interferon Cytokine Res. 2009;29:313–326. doi: 10.1089/jir.2008.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Detry B, Erpicum C, Paupert J, Blacher S, Maillard C, Bruyere F, Pendeville H, Remacle T, Lambert V, Balsat C, Ormenese S, Lamaye F, Janssens E, Moons L, Cataldo D, Kridelka F, Carmeliet P, Thiry M, Foidart JM, Struman I, Noel A. Matrix metalloproteinase-2 governs lymphatic vessel formation as an interstitial collagenase. Blood. 2012;119:5048–5056. doi: 10.1182/blood-2011-12-400267. [DOI] [PubMed] [Google Scholar]
- 65.Dey A, Allen J, Hankey-Giblin PA. Ontogeny and polarization of macrophages in inflammation: blood monocytes versus tissue macrophages. Front Immunol. 2014;5:683. doi: 10.3389/fimmu.2014.00683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dineen SP, Lynn KD, Holloway SE, Miller AF, Sullivan JP, Shames DS, Beck AW, Barnett CC, Fleming JB, Brekken RA. Vascular endothelial growth factor receptor 2 mediates macrophage infiltration into orthotopic pancreatic tumors in mice. Cancer Res. 2008;68:4340–4346. doi: 10.1158/0008-5472.CAN-07-6705. [DOI] [PubMed] [Google Scholar]
- 67.Ding M, Fu X, Tan H, Wang R, Chen Z, Ding S. The effect of vascular endothelial growth factor C expression in tumor-associated macrophages on lymphangiogenesis and lymphatic metastasis in breast cancer. Mol Med Rep. 2012;6:1023–1029. doi: 10.3892/mmr.2012.1043. [DOI] [PubMed] [Google Scholar]
- 68.Ding Y, Song N, Luo Y. Role of bone marrow-derived cells in angiogenesis: focus on macrophages and pericytes. Cancer microenvironment: official journal of the International Cancer Microenvironment Society. 2012;5:225–236. doi: 10.1007/s12307-012-0106-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Diserbo M, Agin A, Lamproglou I, Mauris J, Staali F, Multon E, Amourette C. Blood-brain barrier permeability after gamma whole-body irradiation: an in vivo microdialysis study. Can J Physiol Pharmacol. 2002;80:670–678. doi: 10.1139/y02-070. [DOI] [PubMed] [Google Scholar]
- 70.Doerschuk CM. Pulmonary Alveolar Proteinosis and Macrophage Transplantation. New England Journal of Medicine. 2015;372:1762–1764. doi: 10.1056/NEJMcibr1413035. [DOI] [PubMed] [Google Scholar]
- 71.Duffield JS, Forbes SJ, Constandinou CM, Clay S, Partolina M, Vuthoori S, Wu S, Lang R, Iredale JP. Selective depletion of macrophages reveals distinct, opposing roles during liver injury and repair. J Clin Invest. 2005;115:56–65. doi: 10.1172/JCI22675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.El-Chemaly S, Malide D, Zudaire E, Ikeda Y, Weinberg BA, Pacheco-Rodriguez G, Rosas IO, Aparicio M, Ren P, MacDonald SD, Wu HP, Nathan SD, Cuttitta F, McCoy JP, Gochuico BR, Moss J. Abnormal lymphangiogenesis in idiopathic pulmonary fibrosis with insights into cellular and molecular mechanisms. Proc Natl Acad Sci U S A. 2009;106:3958–3963. doi: 10.1073/pnas.0813368106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Eliska O, Eliskova M. Contribution to the solution of the question of lympho-venous anastomoses in heart of dog. Lymphology. 1975;8:11–15. [PubMed] [Google Scholar]
- 74.Epelman S, Lavine KJ, Randolph GJ. Origin and functions of tissue macrophages. Immunity. 2014;41:21–35. doi: 10.1016/j.immuni.2014.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fadini GP. A reappraisal of the role of circulating (progenitor) cells in the pathobiology of diabetic complications. Diabetologia. 2014;57:4–15. doi: 10.1007/s00125-013-3087-6. [DOI] [PubMed] [Google Scholar]
- 76.Falcone DJ, Khan KMF, Layne T, Fernandes L. Macrophage Formation of Angiostatin during Inflammation: A BYPRODUCT OF THE ACTIVATION OF PLASMINOGEN. Journal of Biological Chemistry. 1998;273:31480–31485. doi: 10.1074/jbc.273.47.31480. [DOI] [PubMed] [Google Scholar]
- 77.Fan Y, Ye J, Shen F, Zhu Y, Yeghiazarians Y, Zhu W, Chen Y, Lawton MT, Young WL, Yang GY. Interleukin-6 stimulates circulating blood-derived endothelial progenitor cell angiogenesis in vitro. J Cereb Blood Flow Metab. 2008;28:90–98. doi: 10.1038/sj.jcbfm.9600509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fantin A, Vieira JM, Gestri G, Denti L, Schwarz Q, Prykhozhij S, Peri F, Wilson SW, Ruhrberg C. Tissue macrophages act as cellular chaperones for vascular anastomosis downstream of VEGF-mediated endothelial tip cell induction. Blood. 2010;116:829–840. doi: 10.1182/blood-2009-12-257832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Farnsworth RH, Lackmann M, Achen MG, Stacker SA. Vascular remodeling in cancer. Oncogene. 2014;33:3496–3505. doi: 10.1038/onc.2013.304. [DOI] [PubMed] [Google Scholar]
- 80.Felcht M, Luck R, Schering A, Seidel P, Srivastava K, Hu J, Bartol A, Kienast Y, Vettel C, Loos EK, Kutschera S, Bartels S, Appak S, Besemfelder E, Terhardt D, Chavakis E, Wieland T, Klein C, Thomas M, Uemura A, Goerdt S, Augustin HG. Angiopoietin-2 differentially regulates angiogenesis through TIE2 and integrin signaling. J Clin Invest. 2012;122:1991–2005. doi: 10.1172/JCI58832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ferrara N, Kerbel RS. Angiogenesis as a therapeutic target. Nature. 2005;438:967–974. doi: 10.1038/nature04483. [DOI] [PubMed] [Google Scholar]
- 82.Ferrari G, Cook BD, Terushkin V, Pintucci G, Mignatti P. Transforming growth factor-beta 1 (TGF-beta1) induces angiogenesis through vascular endothelial growth factor (VEGF)-mediated apoptosis. J Cell Physiol. 2009;219:449–458. doi: 10.1002/jcp.21706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Folkman J. The role of angiogenesis in tumor growth. Seminars in cancer biology. 1992;3:65–71. [PubMed] [Google Scholar]
- 84.Fordham JB, Hua J, Morwood SR, Schewitz-Bowers LP, Copland DA, Dick AD, Nicholson LB. Environmental conditioning in the control of macrophage thrombospondin-1 production. Sci Rep. 2012;2:512. doi: 10.1038/srep00512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Forouzan O, Burns JM, Robichaux JL, Murfee WL, Shevkoplyas SS. Passive recruitment of circulating leukocytes into capillary sprouts from existing capillaries in a microfluidic system. Lab on a chip. 2011;11:1924–1932. doi: 10.1039/c0lc00547a. [DOI] [PubMed] [Google Scholar]
- 86.Fox SB, Moghaddam A, Westwood M, Turley H, Bicknell R, Gatter KC, Harris AL. Platelet-derived endothelial cell growth factor/thymidine phosphorylase expression in normal tissues: an immunohistochemical study. J Pathol. 1995;176:183–190. doi: 10.1002/path.1711760212. [DOI] [PubMed] [Google Scholar]
- 87.Fridlender ZG, Jassar A, Mishalian I, Wang LC, Kapoor V, Cheng G, Sun J, Singhal S, Levy L, Albelda SM. Using macrophage activation to augment immunotherapy of established tumours. Br J Cancer. 2013;108:1288–1297. doi: 10.1038/bjc.2013.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fu J, Gerhardt H, McDaniel JM, Xia B, Liu X, Ivanciu L, Ny A, Hermans K, Silasi-Mansat R, McGee S, Nye E, Ju T, Ramirez MI, Carmeliet P, Cummings RD, Lupu F, Xia L. Endothelial cell O-glycan deficiency causes blood/lymphatic misconnections and consequent fatty liver disease in mice. J Clin Invest. 2008;118:3725–3737. doi: 10.1172/JCI36077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Fujiu K, Wang J, Nagai R. Cardioprotective function of cardiac macrophages. Cardiovasc Res. 2014;102:232–239. doi: 10.1093/cvr/cvu059. [DOI] [PubMed] [Google Scholar]
- 90.Galvan-Pena S, O’Neill LA. Metabolic reprograming in macrophage polarization. Front Immunol. 2014;5:420. doi: 10.3389/fimmu.2014.00420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gamble JR, Elliott MJ, Jaipargas E, Lopez AF, Vadas MA. Regulation of human monocyte adherence by granulocyte-macrophage colony-stimulating factor. Proc Natl Acad Sci U S A. 1989;86:7169–7173. doi: 10.1073/pnas.86.18.7169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gheryani N, Coffelt SB, Gartland A, Rumney RM, Kiss-Toth E, Lewis CE, Tozer GM, Greaves DR, Dear TN, Miller G. Generation of a novel mouse model for the inducible depletion of macrophages in vivo. Genesis. 2013;51:41–49. doi: 10.1002/dvg.22343. [DOI] [PubMed] [Google Scholar]
- 93.Ginhoux F, Jung S. Monocytes and macrophages: developmental pathways and tissue homeostasis. Nat Rev Immunol. 2014;14:392–404. doi: 10.1038/nri3671. [DOI] [PubMed] [Google Scholar]
- 94.Goldmann T, Wieghofer P, Muller PF, Wolf Y, Varol D, Yona S, Brendecke SM, Kierdorf K, Staszewski O, Datta M, Luedde T, Heikenwalder M, Jung S, Prinz M. A new type of microglia gene targeting shows TAK1 to be pivotal in CNS autoimmune inflammation. Nat Neurosci. 2013;16:1618–1626. doi: 10.1038/nn.3531. [DOI] [PubMed] [Google Scholar]
- 95.Gomes FG, Nedel F, Alves AM, Nor JE, Tarquinio SB. Tumor angiogenesis and lymphangiogenesis: tumor/endothelial crosstalk and cellular/microenvironmental signaling mechanisms. Life sciences. 2013;92:101–107. doi: 10.1016/j.lfs.2012.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3:23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- 97.Goren I, Allmann N, Yogev N, Schurmann C, Linke A, Holdener M, Waisman A, Pfeilschifter J, Frank S. A transgenic mouse model of inducible macrophage depletion: effects of diphtheria toxin-driven lysozyme M-specific cell lineage ablation on wound inflammatory, angiogenic, and contractive processes. Am J Pathol. 2009;175:132–147. doi: 10.2353/ajpath.2009.081002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Grundmann S, Schirmer SH, Hekking LH, Post JA, Ionita MG, de Groot D, van Royen N, van den Berg B, Vink H, Moser M, Bode C, de Kleijn D, Pasterkamp G, Piek JJ, Hoefer IE. Endothelial glycocalyx dimensions are reduced in growing collateral arteries and modulate leucocyte adhesion in arteriogenesis. Journal of cellular and molecular medicine. 2009;13:3463–3474. doi: 10.1111/j.1582-4934.2009.00735.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Guha D, Klamar CR, Reinhart T, Ayyavoo V. Transcriptional Regulation of CXCL5 in HIV-1-Infected Macrophages and Its Functional Consequences on CNS Pathology. J Interferon Cytokine Res. 2015;35:373–384. doi: 10.1089/jir.2014.0135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Guillemin GJ, Brew BJ. Microglia, macrophages, perivascular macrophages, and pericytes: a review of function and identification. J Leukoc Biol. 2004;75:388–397. doi: 10.1189/jlb.0303114. [DOI] [PubMed] [Google Scholar]
- 101.Guilliams M, Ginhoux F, Jakubzick C, Naik SH, Onai N, Schraml BU, Segura E, Tussiwand R, Yona S. Dendritic cells, monocytes and macrophages: a unified nomenclature based on ontogeny. Nat Rev Immunol. 2014;14:571–578. doi: 10.1038/nri3712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hall KL, Volk-Draper LD, Flister MJ, Ran S. New model of macrophage acquisition of the lymphatic endothelial phenotype. PLoS One. 2012;7:e31794. doi: 10.1371/journal.pone.0031794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hammer T, Tritsaris K, Hubschmann MV, Gibson J, Nisato RE, Pepper MS, Dissing S. IL-20 activates human lymphatic endothelial cells causing cell signalling and tube formation. Microvasc Res. 2009;78:25–32. doi: 10.1016/j.mvr.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 104.Hao NB, Lu MH, Fan YH, Cao YL, Zhang ZR, Yang SM. Macrophages in tumor microenvironments and the progression of tumors. Clin Dev Immunol. 2012;2012:948098. doi: 10.1155/2012/948098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hashimoto D, Chow A, Noizat C, Teo P, Beasley MB, Leboeuf M, Becker CD, See P, Price J, Lucas D, Greter M, Mortha A, Boyer SW, Forsberg EC, Tanaka M, van Rooijen N, Garcia-Sastre A, Stanley ER, Ginhoux F, Frenette PS, Merad M. Tissue-resident macrophages self-maintain locally throughout adult life with minimal contribution from circulating monocytes. Immunity. 2013;38:792–804. doi: 10.1016/j.immuni.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hawkes CA, McLaurin J. Selective targeting of perivascular macrophages for clearance of beta-amyloid in cerebral amyloid angiopathy. Proc Natl Acad Sci U S A. 2009;106:1261–1266. doi: 10.1073/pnas.0805453106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hayakawa J, Migita M, Ueda T, Shimada T, Fukunaga Y. Generation of a chimeric mouse reconstituted with green fluorescent protein-positive bone marrow cells: a useful model for studying the behavior of bone marrow cells in regeneration in vivo. Int J Hematol. 2003;77:456–462. doi: 10.1007/BF02986613. [DOI] [PubMed] [Google Scholar]
- 108.Heil M, Ziegelhoeffer T, Wagner S, Fernandez B, Helisch A, Martin S, Tribulova S, Kuziel WA, Bachmann G, Schaper W. Collateral artery growth (arteriogenesis) after experimental arterial occlusion is impaired in mice lacking CC-chemokine receptor-2. Circulation research. 2004;94:671–677. doi: 10.1161/01.RES.0000122041.73808.B5. [DOI] [PubMed] [Google Scholar]
- 109.Heppner FL, Greter M, Marino D, Falsig J, Raivich G, Hovelmeyer N, Waisman A, Rulicke T, Prinz M, Priller J, Becher B, Aguzzi A. Experimental autoimmune encephalomyelitis repressed by microglial paralysis. Nat Med. 2005;11:146–152. doi: 10.1038/nm1177. [DOI] [PubMed] [Google Scholar]
- 110.Heuze-Vourc’h N, Liu M, Dalwadi H, Baratelli FE, Zhu L, Goodglick L, Pold M, Sharma S, Ramirez RD, Shay JW, Minna JD, Strieter RM, Dubinett SM. IL-20, an anti-angiogenic cytokine that inhibits COX-2 expression. Biochem Biophys Res Commun. 2005;333:470–475. doi: 10.1016/j.bbrc.2005.05.122. [DOI] [PubMed] [Google Scholar]
- 111.Hirai S, Naito M, Terayama H, Qu N, Kuerban M, Musha M, Itoh M. Lymphangiogenesis in chronic inflammation in the testis. Andrology. 2013;1:147–154. doi: 10.1111/j.2047-2927.2012.00015.x. [DOI] [PubMed] [Google Scholar]
- 112.Höckel M, Jung W, Vaupel P, Rabes H, Khaledpour C, Wissler JH. Purified monocyte-derived angiogenic substance (angiotropin) induces controlled angiogenesis associated with regulated tissue proliferation in rabbit skin. Journal of Clinical Investigation. 1988;82:1075–1090. doi: 10.1172/JCI113664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Höckel M, Sasse J, Wissler JH. Purified monocyte-derived angiogenic substance (angiotropin) stimulates migration, phenotypic changes, and “tube formation” but not proliferation of capillary endothelial cells in vitro. Journal of Cellular Physiology. 1987;133:1–13. doi: 10.1002/jcp.1041330102. [DOI] [PubMed] [Google Scholar]
- 114.Hoeffel G, Chen J, Lavin Y, Low D, Almeida FF, See P, Beaudin AE, Lum J, Low I, Forsberg EC, Poidinger M, Zolezzi F, Larbi A, Ng LG, Chan JK, Greter M, Becher B, Samokhvalov IM, Merad M, Ginhoux F. C-Myb(+) erythro-myeloid progenitor-derived fetal monocytes give rise to adult tissue-resident macrophages. Immunity. 2015;42:665–678. doi: 10.1016/j.immuni.2015.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Horrevoets AJ. Angiogenic monocytes: another colorful blow to endothelial progenitors. Am J Pathol. 2009;174:1594–1596. doi: 10.2353/ajpath.2009.090198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hsieh CL, Kim CC, Ryba BE, Niemi EC, Bando JK, Locksley RM, Liu J, Nakamura MC, Seaman WE. Traumatic brain injury induces macrophage subsets in the brain. Eur J Immunol. 2013;43:2010–2022. doi: 10.1002/eji.201243084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hsieh MY, Chen WY, Jiang MJ, Cheng BC, Huang TY, Chang MS. Interleukin-20 promotes angiogenesis in a direct and indirect manner. Genes Immun. 2006;7:234–242. doi: 10.1038/sj.gene.6364291. [DOI] [PubMed] [Google Scholar]
- 118.Hsu CW, Poche RA, Saik JE, Ali S, Wang S, Yosef N, Calderon GA, Scott L, Jr, Vadakkan TJ, Larina IV, West JL, Dickinson ME. Improved Angiogenesis in Response to Localized Delivery of Macrophage-Recruiting Molecules. PLoS One. 2015;10:e0131643. doi: 10.1371/journal.pone.0131643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hubbard NE, Lim D, Mukutmoni M, Cai A, Erickson KL. Expression and regulation of murine macrophage angiopoietin-2. Cell Immunol. 2005;234:102–109. doi: 10.1016/j.cellimm.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 120.Hume DA. Applications of myeloid-specific promoters in transgenic mice support in vivo imaging and functional genomics but do not support the concept of distinct macrophage and dendritic cell lineages or roles in immunity. Journal of Leukocyte Biology. 2011;89:525–538. doi: 10.1189/jlb.0810472. [DOI] [PubMed] [Google Scholar]
- 121.Iqbal AJ, McNeill E, Kapellos TS, Regan-Komito D, Norman S, Burd S, Smart N, Machemer DEW, Stylianou E, McShane H, Channon KM, Chawla A, Greaves DR. Human CD68 promoter GFP transgenic mice allow analysis of monocyte to macrophage differentiation in vivo. Blood. 2014;124:e33–e44. doi: 10.1182/blood-2014-04-568691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Italiani P, Boraschi D. From Monocytes to M1/M2 Macrophages: Phenotypical vs. Functional Differentiation. Front Immunol. 2014;5:514. doi: 10.3389/fimmu.2014.00514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ito WD, Arras M, Winkler B, Scholz D, Schaper J, Schaper W. Monocyte chemotactic protein-1 increases collateral and peripheral conductance after femoral artery occlusion. Circulation research. 1997;80:829–837. doi: 10.1161/01.res.80.6.829. [DOI] [PubMed] [Google Scholar]
- 124.Jackowski S, Janusch M, Fiedler E, Marsch WC, Ulbrich EJ, Gaisbauer G, Dunst J, Kerjaschki D, Helmbold P. Radiogenic lymphangiogenesis in the skin. Am J Pathol. 2007;171:338–348. doi: 10.2353/ajpath.2007.060589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Jain RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science (New York, NY ) 2005;307:58–62. doi: 10.1126/science.1104819. [DOI] [PubMed] [Google Scholar]
- 126.Jain RK. Antiangiogenesis strategies revisited: from starving tumors to alleviating hypoxia. Cancer Cell. 2014;26:605–622. doi: 10.1016/j.ccell.2014.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Jakubzick C, Gautier EL, Gibbings SL, Sojka DK, Schlitzer A, Johnson TE, Ivanov S, Duan Q, Bala S, Condon T, van Rooijen N, Grainger JR, Belkaid Y, Ma’ayan A, Riches DW, Yokoyama WM, Ginhoux F, Henson PM, Randolph GJ. Minimal differentiation of classical monocytes as they survey steady-state tissues and transport antigen to lymph nodes. Immunity. 2013;39:599–610. doi: 10.1016/j.immuni.2013.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.James JM, Nalbandian A, Mukouyama YS. TGFbeta signaling is required for sprouting lymphangiogenesis during lymphatic network development in the skin. Development. 2013;140:3903–3914. doi: 10.1242/dev.095026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Jauhiainen S, Hakkinen SK, Toivanen PI, Heinonen SE, Jyrkkanen HK, Kansanen E, Leinonen H, Levonen AL, Yla-Herttuala S. Vascular endothelial growth factor (VEGF)-D stimulates VEGF-A, stanniocalcin-1, and neuropilin-2 and has potent angiogenic effects. Arterioscler Thromb Vasc Biol. 2011;31:1617–1624. doi: 10.1161/ATVBAHA.111.225961. [DOI] [PubMed] [Google Scholar]
- 130.Jeon BH, Jang C, Han J, Kataru RP, Piao L, Jung K, Cha HJ, Schwendener RA, Jang KY, Kim KS, Alitalo K, Koh GY. Profound but dysfunctional lymphangiogenesis via vascular endothelial growth factor ligands from CD11b+ macrophages in advanced ovarian cancer. Cancer Res. 2008;68:1100–1109. doi: 10.1158/0008-5472.CAN-07-2572. [DOI] [PubMed] [Google Scholar]
- 131.Ji H, Cao R, Yang Y, Zhang Y, Iwamoto H, Lim S, Nakamura M, Andersson P, Wang J, Sun Y, Dissing S, He X, Yang X, Cao Y. TNFR1 mediates TNF-alpha-induced tumour lymphangiogenesis and metastasis by modulating VEGF-C-VEGFR3 signalling. Nature communications. 2014;5:4944. doi: 10.1038/ncomms5944. [DOI] [PubMed] [Google Scholar]
- 132.Johnson NC, Dillard ME, Baluk P, McDonald DM, Harvey NL, Frase SL, Oliver G. Lymphatic endothelial cell identity is reversible and its maintenance requires Prox1 activity. Genes & development. 2008;22:3282–3291. doi: 10.1101/gad.1727208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Jung JI, Cho HJ, Jung YJ, Kwon SH, Her S, Choi SS, Shin SH, Lee KW, Park JH. High-fat diet-induced obesity increases lymphangiogenesis and lymph node metastasis in the B16F10 melanoma allograft model: roles of adipocytes and M2-macrophages. Int J Cancer. 2015;136:258–270. doi: 10.1002/ijc.28983. [DOI] [PubMed] [Google Scholar]
- 134.Jung S, Aliberti J, Graemmel P, Sunshine MJ, Kreutzberg GW, Sher A, Littman DR. Analysis of Fractalkine Receptor CX(3)CR1 Function by Targeted Deletion and Green Fluorescent Protein Reporter Gene Insertion. Molecular and Cellular Biology. 2000;20:4106–4114. doi: 10.1128/mcb.20.11.4106-4114.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kang S, Lee SP, Kim KE, Kim HZ, Memet S, Koh GY. Toll-like receptor 4 in lymphatic endothelial cells contributes to LPS-induced lymphangiogenesis by chemotactic recruitment of macrophages. Blood. 2009;113:2605–2613. doi: 10.1182/blood-2008-07-166934. [DOI] [PubMed] [Google Scholar]
- 136.Karnevi E, Andersson R, Rosendahl AH. Tumour-educated macrophages display a mixed polarisation and enhance pancreatic cancer cell invasion. Immunol Cell Biol. 2014;92:543–552. doi: 10.1038/icb.2014.22. [DOI] [PubMed] [Google Scholar]
- 137.Karpanen T, Makinen T. Regulation of lymphangiogenesis--from cell fate determination to vessel remodeling. Experimental cell research. 2006;312:575–583. doi: 10.1016/j.yexcr.2005.10.034. [DOI] [PubMed] [Google Scholar]
- 138.Kataru RP, Jung K, Jang C, Yang H, Schwendener RA, Baik JE, Han SH, Alitalo K, Koh GY. Critical role of CD11b+ macrophages and VEGF in inflammatory lymphangiogenesis, antigen clearance, and inflammation resolution. Blood. 2009;113:5650–5659. doi: 10.1182/blood-2008-09-176776. [DOI] [PubMed] [Google Scholar]
- 139.Kawasaki N, Vela JL, Nycholat CM, Rademacher C, Khurana A, van Rooijen N, Crocker PR, Kronenberg M, Paulson JC. Targeted delivery of lipid antigen to macrophages via the CD169/sialoadhesin endocytic pathway induces robust invariant natural killer T cell activation. Proc Natl Acad Sci U S A. 2013;110:7826–7831. doi: 10.1073/pnas.1219888110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kaya M, Palanduz A, Kalayci R, Kemikler G, Simsek G, Bilgic B, Ahishali B, Arican N, Kocyildiz ZC, Elmas I, Kucuk M, Karadeniz A. Effects of lipopolysaccharide on the radiation-induced changes in the blood-brain barrier and the astrocytes. Brain Res. 2004;1019:105–112. doi: 10.1016/j.brainres.2004.05.102. [DOI] [PubMed] [Google Scholar]
- 141.Kelley PM, Connor AL, Tempero RM. Lymphatic vessel memory stimulated by recurrent inflammation. Am J Pathol. 2013;182:2418–2428. doi: 10.1016/j.ajpath.2013.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kelly-Goss MR, Sweat RS, Azimi MS, Murfee WL. Vascular islands during microvascular regression and regrowth in adult networks. Front Physiol. 2013;4:108. doi: 10.3389/fphys.2013.00108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Kelly-Goss MR, Sweat RS, Stapor PC, Peirce SM, Murfee WL. Targeting pericytes for angiogenic therapies. Microcirculation (New York, NY : 1994) 2014;21:345–357. doi: 10.1111/micc.12107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kelly-Goss MR, Winterer ER, Stapor PC, Yang M, Sweat RS, Stallcup WB, Schmid-Schonbein GW, Murfee WL. Cell proliferation along vascular islands during microvascular network growth. BMC physiology. 2012;12:7. doi: 10.1186/1472-6793-12-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kelly C, Jefferies C, Cryan SA. Targeted liposomal drug delivery to monocytes and macrophages. Journal of drug delivery. 2011;2011:727241. doi: 10.1155/2011/727241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kierdorf K, Katzmarski N, Haas CA, Prinz M. Bone marrow cell recruitment to the brain in the absence of irradiation or parabiosis bias. PLoS One. 2013;8:e58544. doi: 10.1371/journal.pone.0058544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Kim OH, Kang GH, Noh H, Cha JY, Lee HJ, Yoon JH, Mamura M, Nam JS, Lee DH, Kim YA, Park YJ, Kim H, Oh BC. Proangiogenic TIE2(+)/CD31 (+) macrophages are the predominant population of tumor-associated macrophages infiltrating metastatic lymph nodes. Mol Cells. 2013;36:432–438. doi: 10.1007/s10059-013-0194-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Kim SJ, Kim JS, Papadopoulos J, Wook Kim S, Maya M, Zhang F, He J, Fan D, Langley R, Fidler IJ. Circulating monocytes expressing CD31: implications for acute and chronic angiogenesis. Am J Pathol. 2009;174:1972–1980. doi: 10.2353/ajpath.2009.080819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Kim WK, Alvarez X, Fisher J, Bronfin B, Westmoreland S, McLaurin J, Williams K. CD163 identifies perivascular macrophages in normal and viral encephalitic brains and potential precursors to perivascular macrophages in blood. Am J Pathol. 2006;168:822–834. doi: 10.2353/ajpath.2006.050215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Kipari T, Cailhier JF, Ferenbach D, Watson S, Houlberg K, Walbaum D, Clay S, Savill J, Hughes J. Nitric oxide is an important mediator of renal tubular epithelial cell death in vitro and in murine experimental hydronephrosis. Am J Pathol. 2006;169:388–399. doi: 10.2353/ajpath.2006.050964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Kraakman MJ, Murphy AJ, Jandeleit-Dahm K, Kammoun HL. Macrophage polarization in obesity and type 2 diabetes: weighing down our understanding of macrophage function? Front Immunol. 2014;5:470. doi: 10.3389/fimmu.2014.00470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Kuan EL, Ivanov S, Bridenbaugh EA, Victora G, Wang W, Childs EW, Platt AM, Jakubzick CV, Mason RJ, Gashev AA, Nussenzweig M, Swartz MA, Dustin ML, Zawieja DC, Randolph GJ. Collecting lymphatic vessel permeability facilitates adipose tissue inflammation and distribution of antigen to lymph node-homing adipose tissue dendritic cells. J Immunol. 2015;194:5200–5210. doi: 10.4049/jimmunol.1500221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Kubota Y, Takubo K, Shimizu T, Ohno H, Kishi K, Shibuya M, Saya H, Suda T. M-CSF inhibition selectively targets pathological angiogenesis and lymphangiogenesis. J Exp Med. 2009;206:1089–1102. doi: 10.1084/jem.20081605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Kunjachan S, Jose S, Thomas CA, Joseph E, Kiessling F, Lammers T. Physicochemical and biological aspects of macrophage-mediated drug targeting in anti-microbial therapy. Fundamental & clinical pharmacology. 2012;26:63–71. doi: 10.1111/j.1472-8206.2011.00955.x. [DOI] [PubMed] [Google Scholar]
- 155.Kurahara H, Takao S, Maemura K, Mataki Y, Kuwahata T, Maeda K, Sakoda M, Iino S, Ishigami S, Ueno S, Shinchi H, Natsugoe S. M2-polarized tumor-associated macrophage infiltration of regional lymph nodes is associated with nodal lymphangiogenesis and occult nodal involvement in pN0 pancreatic cancer. Pancreas. 2013;42:155–159. doi: 10.1097/MPA.0b013e318254f2d1. [DOI] [PubMed] [Google Scholar]
- 156.Kurotaki D, Uede T, Tamura T. Functions and development of red pulp macrophages. Microbiol Immunol. 2015;59:55–62. doi: 10.1111/1348-0421.12228. [DOI] [PubMed] [Google Scholar]
- 157.Kuwahara G, Nishinakamura H, Kojima D, Tashiro T, Kodama S. GM-CSF treated F4/80+ BMCs improve murine hind limb ischemia similar to M-CSF differentiated macrophages. PLoS One. 2014;9:e106987. doi: 10.1371/journal.pone.0106987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Lavin Y, Winter D, Blecher-Gonen R, David E, Keren-Shaul H, Merad M, Jung S, Amit I. Tissue-resident macrophage enhancer landscapes are shaped by the local microenvironment. Cell. 2014;159:1312–1326. doi: 10.1016/j.cell.2014.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Lawler PR, Lawler J. Molecular basis for the regulation of angiogenesis by thrombospondin-1 and -2. Cold Spring Harb Perspect Med. 2012;2:a006627. doi: 10.1101/cshperspect.a006627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Lee M, Aoki M, Kondo T, Kobayashi K, Okumura K, Komori K, Murohara T. Therapeutic angiogenesis with intramuscular injection of low-dose recombinant granulocyte-colony stimulating factor. Arterioscler Thromb Vasc Biol. 2005;25:2535–2541. doi: 10.1161/01.ATV.0000190609.28293.17. [DOI] [PubMed] [Google Scholar]
- 161.Lee SJ, Park C, Lee JY, Kim S, Kwon PJ, Kim W, Jeon YH, Lee E, Yoon YS. Generation of pure lymphatic endothelial cells from human pluripotent stem cells and their therapeutic effects on wound repair. Sci Rep. 2015;5:11019. doi: 10.1038/srep11019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Leibovich SJ, Polverini PJ, Shepard HM, Wiseman DM, Shively V, Nuseir N. Macrophage-induced angiogenesis is mediated by tumour necrosis factor-alpha. Nature. 1987;329:630–632. doi: 10.1038/329630a0. [DOI] [PubMed] [Google Scholar]
- 163.Letterio JJ, Roberts AB. REGULATION OF IMMUNE RESPONSES BY TGF-β*. Annual Review of Immunology. 1998;16:137–161. doi: 10.1146/annurev.immunol.16.1.137. [DOI] [PubMed] [Google Scholar]
- 164.Lewis CE, Pollard JW. Distinct role of macrophages in different tumor microenvironments. Cancer Res. 2006;66:605–612. doi: 10.1158/0008-5472.CAN-05-4005. [DOI] [PubMed] [Google Scholar]
- 165.Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol. 2007;7:678–689. doi: 10.1038/nri2156. [DOI] [PubMed] [Google Scholar]
- 166.Li A, King J, Moro A, Sugi MD, Dawson DW, Kaplan J, Li G, Lu X, Strieter RM, Burdick M, Go VL, Reber HA, Eibl G, Hines OJ. Overexpression of CXCL5 is associated with poor survival in patients with pancreatic cancer. Am J Pathol. 2011;178:1340–1349. doi: 10.1016/j.ajpath.2010.11.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Liao S, Padera TP. Lymphatic function and immune regulation in health and disease. Lymphatic research and biology. 2013;11:136–143. doi: 10.1089/lrb.2013.0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Lin CI, Chen CN, Huang MT, Lee SJ, Lin CH, Chang CC, Lee H. Lysophosphatidic acid up-regulates vascular endothelial growth factor-C and lymphatic marker expressions in human endothelial cells. Cellular and molecular life sciences: CMLS. 2008;65:2740–2751. doi: 10.1007/s00018-008-8314-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Lin EY, Nguyen AV, Russell RG, Pollard JW. Colony-stimulating factor 1 promotes progression of mammary tumors to malignancy. J Exp Med. 2001;193:727–740. doi: 10.1084/jem.193.6.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Liu Z, Condello C, Schain A, Harb R, Grutzendler J. CX3CR1 in microglia regulates brain amyloid deposition through selective protofibrillar amyloid-beta phagocytosis. J Neurosci. 2010;30:17091–17101. doi: 10.1523/JNEUROSCI.4403-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Lobov IB, Rao S, Carroll TJ, Vallance JE, Ito M, Ondr JK, Kurup S, Glass DA, Patel MS, Shu W, Morrisey EE, McMahon AP, Karsenty G, Lang RA. WNT7b mediates macrophage-induced programmed cell death in patterning of the vasculature. Nature. 2005;437:417–421. doi: 10.1038/nature03928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Lucas T, Waisman A, Ranjan R, Roes J, Krieg T, Muller W, Roers A, Eming SA. Differential roles of macrophages in diverse phases of skin repair. J Immunol. 2010;184:3964–3977. doi: 10.4049/jimmunol.0903356. [DOI] [PubMed] [Google Scholar]
- 173.MacDonald KP, Palmer JS, Cronau S, Seppanen E, Olver S, Raffelt NC, Kuns R, Pettit AR, Clouston A, Wainwright B, Branstetter D, Smith J, Paxton RJ, Cerretti DP, Bonham L, Hill GR, Hume DA. An antibody against the colony-stimulating factor 1 receptor depletes the resident subset of monocytes and tissue- and tumor-associated macrophages but does not inhibit inflammation. Blood. 2010;116:3955–3963. doi: 10.1182/blood-2010-02-266296. [DOI] [PubMed] [Google Scholar]
- 174.Machnik A, Neuhofer W, Jantsch J, Dahlmann A, Tammela T, Machura K, Park JK, Beck FX, Muller DN, Derer W, Goss J, Ziomber A, Dietsch P, Wagner H, van Rooijen N, Kurtz A, Hilgers KF, Alitalo K, Eckardt KU, Luft FC, Kerjaschki D, Titze J. Macrophages regulate salt-dependent volume and blood pressure by a vascular endothelial growth factor-C-dependent buffering mechanism. Nat Med. 2009;15:545–552. doi: 10.1038/nm.1960. [DOI] [PubMed] [Google Scholar]
- 175.Malissen B, Tamoutounour S, Henri S. The origins and functions of dendritic cells and macrophages in the skin. Nat Rev Immunol. 2014;14:417–428. doi: 10.1038/nri3683. [DOI] [PubMed] [Google Scholar]
- 176.Malm TM, Koistinaho M, Parepalo M, Vatanen T, Ooka A, Karlsson S, Koistinaho J. Bone-marrow-derived cells contribute to the recruitment of microglial cells in response to beta-amyloid deposition in APP/PS1 double transgenic Alzheimer mice. Neurobiol Dis. 2005;18:134–142. doi: 10.1016/j.nbd.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 177.Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002;23:549–555. doi: 10.1016/s1471-4906(02)02302-5. [DOI] [PubMed] [Google Scholar]
- 178.Martinez FO, Gordon S. The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000Prime Rep. 2014;6:13. doi: 10.12703/P6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Maruyama K, Ii M, Cursiefen C, Jackson DG, Keino H, Tomita M, Van Rooijen N, Takenaka H, D’Amore PA, Stein-Streilein J, Losordo DW, Streilein JW. Inflammation-induced lymphangiogenesis in the cornea arises from CD11b-positive macrophages. J Clin Invest. 2005;115:2363–2372. doi: 10.1172/JCI23874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Matsunaga T. Angiostatin Inhibits Coronary Angiogenesis During Impaired Production of Nitric Oxide. Circulation. 2002;105:2185–2191. doi: 10.1161/01.cir.0000015856.84385.e9. [DOI] [PubMed] [Google Scholar]
- 181.Medina RJ, O’Neill CL, O’Doherty TM, Knott H, Guduric-Fuchs J, Gardiner TA, Stitt AW. Myeloid angiogenic cells act as alternative M2 macrophages and modulate angiogenesis through interleukin-8. Molecular medicine (Cambridge, Mass ) 2011;17:1045–1055. doi: 10.2119/molmed.2011.00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Mendel TA, Clabough EB, Kao DS, Demidova-Rice TN, Durham JT, Zotter BC, Seaman SA, Cronk SM, Rakoczy EP, Katz AJ, Herman IM, Peirce SM, Yates PA. Pericytes derived from adipose-derived stem cells protect against retinal vasculopathy. PLoS One. 2013;8:e65691. doi: 10.1371/journal.pone.0065691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Mills CD, Lenz LL, Ley K. Macrophages at the fork in the road to health or disease. Front Immunol. 2015;6:59. doi: 10.3389/fimmu.2015.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Moldovan NI. Role of monocytes and macrophages in adult angiogenesis: a light at the tunnel’s end. Journal of hematotherapy & stem cell research. 2002;11:179–194. doi: 10.1089/152581602753658394. [DOI] [PubMed] [Google Scholar]
- 185.Mooney JE, Summers KM, Gongora M, Grimmond SM, Campbell JH, Hume DA, Rolfe BE. Transcriptional switching in macrophages associated with the peritoneal foreign body response. Immunol Cell Biol. 2014;92:518–526. doi: 10.1038/icb.2014.19. [DOI] [PubMed] [Google Scholar]
- 186.Morris ME, Beare JE, Reed RM, Dale JR, LeBlanc AJ, Kaufman CL, Zheng H, Ng CK, Williams SK, Hoying JB. Systemically delivered adipose stromal vascular fraction cells disseminate to peripheral artery walls and reduce vasomotor tone through a CD11b+ cell-dependent mechanism. Stem cells translational medicine. 2015;4:369–380. doi: 10.5966/sctm.2014-0252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Moussai D, Mitsui H, Pettersen JS, Pierson KC, Shah KR, Suarez-Farinas M, Cardinale IR, Bluth MJ, Krueger JG, Carucci JA. The human cutaneous squamous cell carcinoma microenvironment is characterized by increased lymphatic density and enhanced expression of macrophage-derived VEGF-C. The Journal of investigative dermatology. 2011;131:229–236. doi: 10.1038/jid.2010.266. [DOI] [PubMed] [Google Scholar]
- 189.Mowat AM, Bain CC. Mucosal macrophages in intestinal homeostasis and inflammation. J Innate Immun. 2011;3:550–564. doi: 10.1159/000329099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Muller PA, Koscso B, Rajani GM, Stevanovic K, Berres ML, Hashimoto D, Mortha A, Leboeuf M, Li XM, Mucida D, Stanley ER, Dahan S, Margolis KG, Gershon MD, Merad M, Bogunovic M. Crosstalk between muscularis macrophages and enteric neurons regulates gastrointestinal motility. Cell. 2014;158:300–313. doi: 10.1016/j.cell.2014.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Munson JM, Shieh AC. Interstitial fluid flow in cancer: implications for disease progression and treatment. Cancer Manag Res. 2014;6:317–328. doi: 10.2147/CMAR.S65444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Murfee WL, Rehorn MR, Peirce SM, Skalak TC. Perivascular cells along venules upregulate NG2 expression during microvascular remodeling. Microcirculation (New York, NY : 1994) 2006;13:261–273. doi: 10.1080/10739680600559153. [DOI] [PubMed] [Google Scholar]
- 193.Murray PJ, Allen JE, Biswas SK, Fisher EA, Gilroy DW, Goerdt S, Gordon S, Hamilton JA, Ivashkiv LB, Lawrence T, Locati M, Mantovani A, Martinez FO, Mege JL, Mosser DM, Natoli G, Saeij JP, Schultze JL, Shirey KA, Sica A, Suttles J, Udalova I, van Ginderachter JA, Vogel SN, Wynn TA. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity. 2014;41:14–20. doi: 10.1016/j.immuni.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Murray PJ, Wynn TA. Protective and pathogenic functions of macrophage subsets. Nat Rev Immunol. 2011;11:723–737. doi: 10.1038/nri3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Myöhänen TT, Tenorio-Laranga J, Jokinen B, Vázquez-Sánchez R, Moreno-Baylach MJ, García-Horsman JA, Männistö PT. Prolyl oligopeptidase induces angiogenesis both in vitro and in vivo in a novel regulatory manner. British Journal of Pharmacology. 2011;163:1666–1678. doi: 10.1111/j.1476-5381.2010.01146.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Nagy JA, Brown LF, Senger DR, Lanir N, Van de Water L, Dvorak AM, Dvorak HF. Pathogenesis of tumor stroma generation: a critical role for leaky blood vessels and fibrin deposition. Biochimica et biophysica acta. 1989;948:305–326. doi: 10.1016/0304-419x(89)90004-8. [DOI] [PubMed] [Google Scholar]
- 197.Natori T, Sata M, Washida M, Hirata Y, Nagai R, Makuuchi M. G-CSF stimulates angiogenesis and promotes tumor growth: potential contribution of bone marrow-derived endothelial progenitor cells. Biochemical and Biophysical Research Communications. 2002;297:1058–1061. doi: 10.1016/s0006-291x(02)02335-5. [DOI] [PubMed] [Google Scholar]
- 198.Nayak D, Roth TL, McGavern DB. Microglia development and function. Annu Rev Immunol. 2014;32:367–402. doi: 10.1146/annurev-immunol-032713-120240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Nguyen KD, Qiu Y, Cui X, Goh YPS, Mwangi J, David T, Mukundan L, Brombacher F, Locksley RM, Chawla A. Alternatively activated macrophages produce catecholamines to sustain adaptive thermogenesis. Nature. 2011;480:104–108. doi: 10.1038/nature10653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Nickerson MM, Burke CW, Meisner JK, Shuptrine CW, Song J, Price RJ. Capillary arterialization requires the bone-marrow-derived cell (BMC)-specific expression of chemokine (C-C motif) receptor-2, but BMCs do not transdifferentiate into microvascular smooth muscle. Angiogenesis. 2009;12:355–363. doi: 10.1007/s10456-009-9157-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Nowak DG, Woolard J, Amin EM, Konopatskaya O, Saleem MA, Churchill AJ, Ladomery MR, Harper SJ, Bates DO. Expression of pro- and anti-angiogenic isoforms of VEGF is differentially regulated by splicing and growth factors. J Cell Sci. 2008;121:3487–3495. doi: 10.1242/jcs.016410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Noy R, Pollard JW. Tumor-associated macrophages: from mechanisms to therapy. Immunity. 2014;41:49–61. doi: 10.1016/j.immuni.2014.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Nucera S, Biziato D, De Palma M. The interplay between macrophages and angiogenesis in development, tissue injury and regeneration. Int J Dev Biol. 2011;55:495–503. doi: 10.1387/ijdb.103227sn. [DOI] [PubMed] [Google Scholar]
- 204.Oh CW, Hoover-Plow J, Plow EF. The role of plasminogen in angiogenesis in vivo. J Thromb Haemost. 2003;1:1683–1687. doi: 10.1046/j.1538-7836.2003.00182.x. [DOI] [PubMed] [Google Scholar]
- 205.Oka M, Iwata C, Suzuki HI, Kiyono K, Morishita Y, Watabe T, Komuro A, Kano MR, Miyazono K. Inhibition of endogenous TGF-β signaling enhances lymphangiogenesis. 2008;111 doi: 10.1182/blood-2007-10-120337. [DOI] [PubMed] [Google Scholar]
- 206.Onishi T, Nishizuka T, Kurahashi T, Arai T, Iwatsuki K, Yamamoto M, Hirata H. Topical bFGF Improves Secondary Lymphedema through Lymphangiogenesis in a Rat Tail Model. Plast Reconstr Surg Glob Open. 2014;2:e196. doi: 10.1097/GOX.0000000000000154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Ozerdem U, Alitalo K, Salven P, Li A. Contribution of bone marrow-derived pericyte precursor cells to corneal vasculogenesis. Investigative ophthalmology & visual science. 2005;46:3502–3506. doi: 10.1167/iovs.05-0309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Pantel K, Alix-Panabieres C, Riethdorf S. Cancer micrometastases. Nat Rev Clin Oncol. 2009;6:339–351. doi: 10.1038/nrclinonc.2009.44. [DOI] [PubMed] [Google Scholar]
- 209.Park YW, Kang YM, Butterfield J, Detmar M, Goronzy JJ, Weyand CM. Thrombospondin 2 Functions as an Endogenous Regulator of Angiogenesis and Inflammation in Rheumatoid Arthritis. The American Journal of Pathology. 2004;165:2087–2098. doi: 10.1016/S0002-9440(10)63259-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Parsons-Wingerter P, McKay TL, Leontiev D, Vickerman MB, Condrich TK, Dicorleto PE. Lymphangiogenesis by blind-ended vessel sprouting is concurrent with hemangiogenesis by vascular splitting. The anatomical record. Part A, Discoveries in molecular, cellular, and evolutionary biology. 2006;288:233–247. doi: 10.1002/ar.a.20309. [DOI] [PubMed] [Google Scholar]
- 211.Peirce SM. Computational and mathematical modeling of angiogenesis. Microcirculation (New York, NY : 1994) 2008;15:739–751. doi: 10.1080/10739680802220331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Phillips GD, Whitehead RA, Stone AM, Ruebel MW, Goodkin ML, Knighton DR. Transforming growth factor beta (TGF-B) stimulation of angiogenesis: an electron microscopic study. Journal of submicroscopic cytology and pathology. 1993;25:149–155. [PubMed] [Google Scholar]
- 213.Poche RA, Hsu CW, McElwee ML, Burns AR, Dickinson ME. Macrophages engulf endothelial cell membrane particles preceding pupillary membrane capillary regression. Developmental biology. 2015;403:30–42. doi: 10.1016/j.ydbio.2015.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Powner MB, Vevis K, McKenzie JA, Gandhi P, Jadeja S, Fruttiger M. Visualization of gene expression in whole mouse retina by in situ hybridization. Nat Protoc. 2012;7:1086–1096. doi: 10.1038/nprot.2012.050. [DOI] [PubMed] [Google Scholar]
- 215.Priller J, Flugel A, Wehner T, Boentert M, Haas CA, Prinz M, Fernandez-Klett F, Prass K, Bechmann I, de Boer BA, Frotscher M, Kreutzberg GW, Persons DA, Dirnagl U. Targeting gene-modified hematopoietic cells to the central nervous system: use of green fluorescent protein uncovers microglial engraftment. Nat Med. 2001;7:1356–1361. doi: 10.1038/nm1201-1356. [DOI] [PubMed] [Google Scholar]
- 216.Priller J, Prinz M, Heikenwalder M, Zeller N, Schwarz P, Heppner FL, Aguzzi A. Early and rapid engraftment of bone marrow-derived microglia in scrapie. J Neurosci. 2006;26:11753–11762. doi: 10.1523/JNEUROSCI.2275-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Prinz M, Priller J. Microglia and brain macrophages in the molecular age: from origin to neuropsychiatric disease. Nat Rev Neurosci. 2014;15:300–312. doi: 10.1038/nrn3722. [DOI] [PubMed] [Google Scholar]
- 218.Prokopi M, Pula G, Mayr U, Devue C, Gallagher J, Xiao Q, Boulanger CM, Westwood N, Urbich C, Willeit J, Steiner M, Breuss J, Xu Q, Kiechl S, Mayr M. Proteomic analysis reveals presence of platelet microparticles in endothelial progenitor cell cultures. Blood. 2009;114:723–732. doi: 10.1182/blood-2009-02-205930. [DOI] [PubMed] [Google Scholar]
- 219.Qiu H, Cao L, Wang D, Xu H, Liang Z. High levels of circulating CD34+/VEGFR3+ lymphatic/vascular endothelial progenitor cells is correlated with lymph node metastasis in patients with epithelial ovarian cancer. J Obstet Gynaecol Res. 2013;39:1268–1275. doi: 10.1111/jog.12047. [DOI] [PubMed] [Google Scholar]
- 220.Raggi C, Mousa HS, Correnti M, Sica A, Invernizzi P. Cancer stem cells and tumor-associated macrophages: a roadmap for multitargeting strategies. Oncogene. 2015 doi: 10.1038/onc.2015.132. [DOI] [PubMed] [Google Scholar]
- 221.Ran S, Montgomery KE. Macrophage-mediated lymphangiogenesis: the emerging role of macrophages as lymphatic endothelial progenitors. Cancers. 2012;4:618–657. doi: 10.3390/cancers4030618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Randolph GJ, Jakubzick C, Qu C. Antigen Presentation by Monocytes and Monocyte-derived Cells. Current opinion in immunology. 2008;20:52–60. doi: 10.1016/j.coi.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Rangasamy S, McGuire PG, Franco Nitta C, Monickaraj F, Oruganti SR, Das A. Chemokine mediated monocyte trafficking into the retina: role of inflammation in alteration of the blood-retinal barrier in diabetic retinopathy. PLoS One. 2014;9:e108508. doi: 10.1371/journal.pone.0108508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Rath M, Muller I, Kropf P, Closs EI, Munder M. Metabolism via Arginase or Nitric Oxide Synthase: Two Competing Arginine Pathways in Macrophages. Front Immunol. 2014;5:532. doi: 10.3389/fimmu.2014.00532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Rehman J, Li J, Orschell CM, March KL. Peripheral blood “endothelial progenitor cells” are derived from monocyte/macrophages and secrete angiogenic growth factors. Circulation. 2003;107:1164–1169. doi: 10.1161/01.cir.0000058702.69484.a0. [DOI] [PubMed] [Google Scholar]
- 226.Religa P, Cao R, Bjorndahl M, Zhou Z, Zhu Z, Cao Y. Presence of bone marrow-derived circulating progenitor endothelial cells in the newly formed lymphatic vessels. Blood. 2005;106:4184–4190. doi: 10.1182/blood-2005-01-0226. [DOI] [PubMed] [Google Scholar]
- 227.Riabov V, Gudima A, Wang N, Mickley A, Orekhov A, Kzhyshkowska J. Role of tumor associated macrophages in tumor angiogenesis and lymphangiogenesis. Front Physiol. 2014;5:75. doi: 10.3389/fphys.2014.00075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Ribatti D, Maruotti N, Nico B, Longo V, Mangieri D, Vacca A, Cantatore FP. Clodronate inhibits angiogenesis in vitro and in vivo. Oncology reports. 2008;19:1109–1112. doi: 10.3892/or.19.5.1109. [DOI] [PubMed] [Google Scholar]
- 229.Richards J, Gabunia K, Kelemen SE, Kako F, Choi ET, Autieri MV. Interleukin-19 increases angiogenesis in ischemic hind limbs by direct effects on both endothelial cells and macrophage polarization. J Mol Cell Cardiol. 2015;79:21–31. doi: 10.1016/j.yjmcc.2014.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Ries CH, Cannarile MA, Hoves S, Benz J, Wartha K, Runza V, Rey-Giraud F, Pradel LP, Feuerhake F, Klaman I, Jones T, Jucknischke U, Scheiblich S, Kaluza K, Gorr IH, Walz A, Abiraj K, Cassier PA, Sica A, Gomez-Roca C, de Visser KE, Italiano A, Le Tourneau C, Delord JP, Levitsky H, Blay JY, Ruttinger D. Targeting tumor-associated macrophages with anti-CSF-1R antibody reveals a strategy for cancer therapy. Cancer Cell. 2014;25:846–859. doi: 10.1016/j.ccr.2014.05.016. [DOI] [PubMed] [Google Scholar]
- 231.Rissanen TT, Markkanen JE, Gruchala M, Heikura T, Puranen A, Kettunen MI, Kholova I, Kauppinen RA, Achen MG, Stacker SA, Alitalo K, Yla-Herttuala S. VEGF-D is the strongest angiogenic and lymphangiogenic effector among VEGFs delivered into skeletal muscle via adenoviruses. Circulation research. 2003;92:1098–1106. doi: 10.1161/01.RES.0000073584.46059.E3. [DOI] [PubMed] [Google Scholar]
- 232.Robichaux JL, Tanno E, Rappleye JW, Ceballos M, Stallcup WB, Schmid-Schonbein GW, Murfee WL. Lymphatic/Blood endothelial cell connections at the capillary level in adult rat mesentery. Anatomical record (Hoboken, NJ : 2007) 2010;293:1629–1638. doi: 10.1002/ar.21195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Rockson SG. Lymphatics: where the circulation meets the immune system. Lymphatic research and biology. 2013;11:115. doi: 10.1089/lrb.2013.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Root RK, Dale DC. Granulocyte Colony-Stimulating Factor and Granulocyte-Macrophage Colony-Stimulating Factor: Comparisons and Potential for Use in the Treatment of Infections in Nonneutropenic Patients. Journal of Infectious Diseases. 1999;179:S342–S352. doi: 10.1086/513857. [DOI] [PubMed] [Google Scholar]
- 235.Roszer T. Understanding the Mysterious M2 Macrophage through Activation Markers and Effector Mechanisms. Mediators Inflamm. 2015;2015:816460. doi: 10.1155/2015/816460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Rőszer T. Understanding the Mysterious M2 Macrophage through Activation Markers and Effector Mechanisms. Mediators of Inflammation. 2015;2015:816460. doi: 10.1155/2015/816460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Rundhaug JE. Matrix metalloproteinases and angiogenesis. Journal of cellular and molecular medicine. 2005;9:267–285. doi: 10.1111/j.1582-4934.2005.tb00355.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Rutkowski JM, Boardman KC, Swartz MA. Characterization of lymphangiogenesis in a model of adult skin regeneration. American journal of physiology. Heart and circulatory physiology. 2006;291:H1402–1410. doi: 10.1152/ajpheart.00038.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Rymo SF, Gerhardt H, Wolfhagen Sand F, Lang R, Uv A, Betsholtz C. A two-way communication between microglial cells and angiogenic sprouts regulates angiogenesis in aortic ring cultures. PLoS One. 2011;6:e15846. doi: 10.1371/journal.pone.0015846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Saederup N, Cardona AE, Croft K, Mizutani M, Cotleur AC, Tsou CL, Ransohoff RM, Charo IF. Selective chemokine receptor usage by central nervous system myeloid cells in CCR2-red fluorescent protein knock-in mice. PLoS One. 2010;5:e13693. doi: 10.1371/journal.pone.0013693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Samokhvalov IM, Samokhvalova NI, Nishikawa S. Cell tracing shows the contribution of the yolk sac to adult haematopoiesis. Nature. 2007;446:1056–1061. doi: 10.1038/nature05725. [DOI] [PubMed] [Google Scholar]
- 242.Sasmono RT, Williams E. Generation and characterization of MacGreen mice, the Cfs1r-EGFP transgenic mice. Methods Mol Biol. 2012;844:157–176. doi: 10.1007/978-1-61779-527-5_11. [DOI] [PubMed] [Google Scholar]
- 243.Sato N, Beitz JG, Kato J, Yamamoto M, Clark JW, Calabresi P, Raymond A, Frackelton AR. Platelet-derived growth factor indirectly stimulates angiogenesis in vitro. The American Journal of Pathology. 1993;142:1119–1130. [PMC free article] [PubMed] [Google Scholar]
- 244.Schaper W. Control of coronary angiogenesis. European heart journal. 1995;16(Suppl C):66–68. doi: 10.1093/eurheartj/16.suppl_c.66. [DOI] [PubMed] [Google Scholar]
- 245.Schledzewski K, Falkowski M, Moldenhauer G, Metharom P, Kzhyshkowska J, Ganss R, Demory A, Falkowska-Hansen B, Kurzen H, Ugurel S, Geginat G, Arnold B, Goerdt S. Lymphatic endothelium-specific hyaluronan receptor LYVE-1 is expressed by stabilin-1+, F4/80+, CD11b+ macrophages in malignant tumours and wound healing tissue in vivo and in bone marrow cultures in vitro: implications for the assessment of lymphangiogenesis. J Pathol. 2006;209:67–77. doi: 10.1002/path.1942. [DOI] [PubMed] [Google Scholar]
- 246.Schnaper HW, Grant DS, Stetler-Stevenson WG, Fridman R, D’Orazi G, Murphy AN, Bird RE, Hoythya M, Fuerst TR, French DL, et al. Type IV collagenase(s) and TIMPs modulate endothelial cell morphogenesis in vitro. J Cell Physiol. 1993;156:235–246. doi: 10.1002/jcp.1041560204. [DOI] [PubMed] [Google Scholar]
- 247.Schoppmann SF, Birner P, Stockl J, Kalt R, Ullrich R, Caucig C, Kriehuber E, Nagy K, Alitalo K, Kerjaschki D. Tumor-associated macrophages express lymphatic endothelial growth factors and are related to peritumoral lymphangiogenesis. Am J Pathol. 2002;161:947–956. doi: 10.1016/S0002-9440(10)64255-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Schoppmann SF, Fenzl A, Nagy K, Unger S, Bayer G, Geleff S, Gnant M, Horvat R, Jakesz R, Birner P. VEGF-C expressing tumor-associated macrophages in lymph node positive breast cancer: impact on lymphangiogenesis and survival. Surgery. 2006;139:839–846. doi: 10.1016/j.surg.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 249.Schreiber HA, Loschko J, Karssemeijer RA, Escolano A, Meredith MM, Mucida D, Guermonprez P, Nussenzweig MC. Intestinal monocytes and macrophages are required for T cell polarization in response to Citrobacter rodentium. J Exp Med. 2013;210:2025–2039. doi: 10.1084/jem.20130903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Shankman LS, Gomez D, Cherepanova OA, Salmon M, Alencar GF, Haskins RM, Swiatlowska P, Newman AA, Greene ES, Straub AC, Isakson B, Randolph GJ, Owens GK. KLF4-dependent phenotypic modulation of smooth muscle cells has a key role in atherosclerotic plaque pathogenesis. Nat Med. 2015;21:628–637. doi: 10.1038/nm.3866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Shao XJ, Xie FM. Influence of angiogenesis inhibitors, endostatin and PF-4, on lymphangiogenesis. Lymphology. 2005;38:1–8. [PubMed] [Google Scholar]
- 252.Shi C, Jia T, Mendez-Ferrer S, Hohl TM, Serbina NV, Lipuma L, Leiner I, Li MO, Frenette PS, Pamer EG. Bone marrow mesenchymal stem and progenitor cells induce monocyte emigration in response to circulating toll-like receptor ligands. Immunity. 2011;34:590–601. doi: 10.1016/j.immuni.2011.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Shi C, Pamer EG. Monocyte recruitment during infection and inflammation. Nat Rev Immunol. 2011;11:762–774. doi: 10.1038/nri3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Shi Y, Liu CH, Roberts AI, Das J, Xu G, Ren G, Zhang Y, Zhang L, Yuan ZR, Tan HS, Das G, Devadas S. Granulocyte-macrophage colony-stimulating factor (GM-CSF) and T-cell responses: what we do and don’t know. Cell Res. 2006;16:126–133. doi: 10.1038/sj.cr.7310017. [DOI] [PubMed] [Google Scholar]
- 255.Shimizu Y, Shibata R, Shintani S, Ishii M, Murohara T. Therapeutic lymphangiogenesis with implantation of adipose-derived regenerative cells. Journal of the American Heart Association. 2012;1:e000877. doi: 10.1161/JAHA.112.000877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Skobe M, Hamberg LM, Hawighorst T, Schirner M, Wolf GL, Alitalo K, Detmar M. Concurrent induction of lymphangiogenesis, angiogenesis, and macrophage recruitment by vascular endothelial growth factor-C in melanoma. Am J Pathol. 2001;159:893–903. doi: 10.1016/S0002-9440(10)61765-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Skobe M, Hawighorst T, Jackson DG, Prevo R, Janes L, Velasco P, Riccardi L, Alitalo K, Claffey K, Detmar M. Induction of tumor lymphangiogenesis by VEGF-C promotes breast cancer metastasis. Nat Med. 2001;7:192–198. doi: 10.1038/84643. [DOI] [PubMed] [Google Scholar]
- 258.Solomon JN, Lewis CA, Ajami B, Corbel SY, Rossi FM, Krieger C. Origin and distribution of bone marrow-derived cells in the central nervous system in a mouse model of amyotrophic lateral sclerosis. Glia. 2006;53:744–753. doi: 10.1002/glia.20331. [DOI] [PubMed] [Google Scholar]
- 259.Spiller KL, Anfang RR, Spiller KJ, Ng J, Nakazawa KR, Daulton JW, Vunjak-Novakovic G. The role of macrophage phenotype in vascularization of tissue engineering scaffolds. Biomaterials. 2014;35:4477–4488. doi: 10.1016/j.biomaterials.2014.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Stacker SA, Achen MG. From anti-angiogenesis to anti-lymphangiogenesis: emerging trends in cancer therapy. Lymphatic research and biology. 2008;6:165–172. doi: 10.1089/lrb.2008.1015. [DOI] [PubMed] [Google Scholar]
- 261.Stacker SA, Williams SP, Karnezis T, Shayan R, Fox SB, Achen MG. Lymphangiogenesis and lymphatic vessel remodelling in cancer. Nature reviews. Cancer. 2014;14:159–172. doi: 10.1038/nrc3677. [DOI] [PubMed] [Google Scholar]
- 262.Stamatovic SM, Keep RF, Mostarica-Stojkovic M, Andjelkovic AV. CCL2 Regulates Angiogenesis via Activation of Ets-1 Transcription Factor. The Journal of Immunology. 2006;177:2651–2661. doi: 10.4049/jimmunol.177.4.2651. [DOI] [PubMed] [Google Scholar]
- 263.Stapor PC, Azimi MS, Ahsan T, Murfee WL. An angiogenesis model for investigating multicellular interactions across intact microvascular networks. American journal of physiology. Heart and circulatory physiology. 2013;304:H235–245. doi: 10.1152/ajpheart.00552.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Stark K, Eckart A, Haidari S, Tirniceriu A, Lorenz M, von Bruhl ML, Gartner F, Khandoga AG, Legate KR, Pless R, Hepper I, Lauber K, Walzog B, Massberg S. Capillary and arteriolar pericytes attract innate leukocytes exiting through venules and ‘instruct’ them with pattern-recognition and motility programs. Nat Immunol. 2013;14:41–51. doi: 10.1038/ni.2477. [DOI] [PubMed] [Google Scholar]
- 265.Stephan J, Melaine N, Ezan E, Hakovirta H, Maddocks S, Toppari J, Garnier D, Wdzieczak-Bakala J, Jegou B. Source, catabolism and role of the tetrapeptide N-acetyl-ser-asp-lys-Pro within the testis. J Cell Sci. 2000;113(Pt 1):113–121. doi: 10.1242/jcs.113.1.113. [DOI] [PubMed] [Google Scholar]
- 266.Stockmann C, Kirmse S, Helfrich I, Weidemann A, Takeda N, Doedens A, Johnson RS. A wound size-dependent effect of myeloid cell-derived vascular endothelial growth factor on wound healing. The Journal of investigative dermatology. 2011;131:797–801. doi: 10.1038/jid.2010.345. [DOI] [PubMed] [Google Scholar]
- 267.Suh H-S, Zhao M-L, Derico L, Choi N, Lee S. Insulin-like growth factor 1 and 2 (IGF1, IGF2) expression in human microglia: differential regulation by inflammatory mediators. Journal of Neuroinflammation. 2013;10:37. doi: 10.1186/1742-2094-10-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Sunderkotter C, Goebeler M, Schulze-Osthoff K, Bhardwaj R, Sorg C. Macrophage-derived angiogenesis factors. Pharmacology & Therapeutics. 1991;51:195–216. doi: 10.1016/0163-7258(91)90077-y. [DOI] [PubMed] [Google Scholar]
- 269.Sunderkötter C, Steinbrink K, Goebeler M, Bhardwaj R, Sorg C. Macrophages and angiogenesis. J Leukoc Biol. 1994;55:410–422. doi: 10.1002/jlb.55.3.410. [DOI] [PubMed] [Google Scholar]
- 270.Suzuki Y, Ito Y, Mizuno M, Kinashi H, Sawai A, Noda Y, Mizuno T, Shimizu H, Fujita Y, Matsui K, Maruyama S, Imai E, Matsuo S, Takei Y. Transforming growth factor-beta induces vascular endothelial growth factor-C expression leading to lymphangiogenesis in rat unilateral ureteral obstruction. Kidney Int. 2012;81:865–879. doi: 10.1038/ki.2011.464. [DOI] [PubMed] [Google Scholar]
- 271.Sweat RS, Sloas DC, Murfee WL. VEGF-C induces lymphangiogenesis and angiogenesis in the rat mesentery culture model. Microcirculation (New York, NY : 1994) 2014;21:532–540. doi: 10.1111/micc.12132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Sweat RS, Stapor PC, Murfee WL. Relationships between lymphangiogenesis and angiogenesis during inflammation in rat mesentery microvascular networks. Lymphatic research and biology. 2012;10:198–207. doi: 10.1089/lrb.2012.0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Swirski FK, Nahrendorf M. Do vascular smooth muscle cells differentiate to macrophages in atherosclerotic lesions? Circulation research. 2014;115:605–606. doi: 10.1161/CIRCRESAHA.114.304925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Takeda K, Sowa Y, Nishino K, Itoh K, Fushiki S. Adipose-derived stem cells promote proliferation, migration, and tube formation of lymphatic endothelial cells in vitro by secreting lymphangiogenic factors. Ann Plast Surg. 2015;74:728–736. doi: 10.1097/SAP.0000000000000084. [DOI] [PubMed] [Google Scholar]
- 275.Tamoutounour S, Guilliams M, Montanana Sanchis F, Liu H, Terhorst D, Malosse C, Pollet E, Ardouin L, Luche H, Sanchez C, Dalod M, Malissen B, Henri S. Origins and functional specialization of macrophages and of conventional and monocyte-derived dendritic cells in mouse skin. Immunity. 2013;39:925–938. doi: 10.1016/j.immuni.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 276.Tauber AI. Metchnikoff and the phagocytosis theory. Nature reviews. Molecular cell biology. 2003;4:897–901. doi: 10.1038/nrm1244. [DOI] [PubMed] [Google Scholar]
- 277.Thomas G, Tacke R, Hedrick CC, Hanna RN. Nonclassical patrolling monocyte function in the vasculature. Arterioscler Thromb Vasc Biol. 2015;35:1306–1316. doi: 10.1161/ATVBAHA.114.304650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Toblli JE, Cao G, DeRosa G, Gennaro FD, Forcada P. Angiotensin-Converting enzyme inhibition and angiogenesis in myocardium of obese Zucker rats. American Journal of Hypertension. 2004;17:172–180. doi: 10.1016/j.amjhyper.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 279.Tura O, Crawford J, Barclay GR, Samuel K, Hadoke PW, Roddie H, Davies J, Turner ML. Granulocyte colony-stimulating factor (G-CSF) depresses angiogenesis in vivo and in vitro: implications for sourcing cells for vascular regeneration therapy. J Thromb Haemost. 2010;8:1614–1623. doi: 10.1111/j.1538-7836.2010.03900.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Urbich C, Heeschen C, Aicher A, Dernbach E, Zeiher AM, Dimmeler S. Relevance of monocytic features for neovascularization capacity of circulating endothelial progenitor cells. Circulation. 2003;108:2511–2516. doi: 10.1161/01.CIR.0000096483.29777.50. [DOI] [PubMed] [Google Scholar]
- 281.Van’t Hull EF, Bron S, Henry L, Ifticene-Treboux A, Turrini R, Coukos G, Delaloye JF, Doucey MA. Bone marrow-derived cells are implicated as a source of lymphatic endothelial progenitors in human breast cancer. Oncoimmunology. 2014;3:e29080. doi: 10.4161/onci.29080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.van Furth R, Cohn ZA. THE ORIGIN AND KINETICS OF MONONUCLEAR PHAGOCYTES. The Journal of Experimental Medicine. 1968;128:415–435. doi: 10.1084/jem.128.3.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.van Furth R, Cohn ZA, Hirsch JG, Humphrey JH, Spector WG, Langevoort HL. The mononuclear phagocyte system: a new classification of macrophages, monocytes, and their precursor cells. Bull World Health Organ. 1972;46:845–852. [PMC free article] [PubMed] [Google Scholar]
- 284.van Helden MJG, Zaiss DMW, Sijts AJAM. CCR2 Defines a Distinct Population of NK Cells and Mediates Their Migration during Influenza Virus Infection in Mice. PLoS ONE. 2012;7:e52027. doi: 10.1371/journal.pone.0052027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.van Rooijen N, Hendrikx E. Liposomes for specific depletion of macrophages from organs and tissues. Methods Mol Biol. 2010;605:189–203. doi: 10.1007/978-1-60327-360-2_13. [DOI] [PubMed] [Google Scholar]
- 286.van Rooijen N, Sanders A. Elimination, blocking, and activation of macrophages: three of a kind? Journal of leukocyte biology. 1997;62:702–709. doi: 10.1002/jlb.62.6.702. [DOI] [PubMed] [Google Scholar]
- 287.Veale D, Yanni G, Bresnihan B, FitzGerald O. Production of angiotensin converting enzyme by rheumatoid synovial membrane. Annals of the Rheumatic Diseases. 1992;51:476–480. doi: 10.1136/ard.51.4.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Venneri MA, De Palma M, Ponzoni M, Pucci F, Scielzo C, Zonari E, Mazzieri R, Doglioni C, Naldini L. Identification of proangiogenic TIE2-expressing monocytes (TEMs) in human peripheral blood and cancer. Blood. 2007;109:5276–5285. doi: 10.1182/blood-2006-10-053504. [DOI] [PubMed] [Google Scholar]
- 289.Volk-Draper L, Hall K, Griggs C, Rajput S, Kohio P, DeNardo D, Ran S. Paclitaxel therapy promotes breast cancer metastasis in a TLR4-dependent manner. Cancer Res. 2014;74:5421–5434. doi: 10.1158/0008-5472.CAN-14-0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Voronov E, Shouval DS, Krelin Y, Cagnano E, Benharroch D, Iwakura Y, Dinarello CA, Apte RN. IL-1 is required for tumor invasiveness and angiogenesis. Proc Natl Acad Sci U S A. 2003;100:2645–2650. doi: 10.1073/pnas.0437939100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Wang B, Zhuang X, Deng ZB, Jiang H, Mu J, Wang Q, Xiang X, Guo H, Zhang L, Dryden G, Yan J, Miller D, Zhang HG. Targeted drug delivery to intestinal macrophages by bioactive nanovesicles released from grapefruit. Molecular therapy: the journal of the American Society of Gene Therapy. 2014;22:522–534. doi: 10.1038/mt.2013.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Wang N, Rajasekaran N, Hou T, Mellins ED. Transgene expression in various organs post BM-HSC transplantation. Stem Cell Res. 2014;12:209–221. doi: 10.1016/j.scr.2013.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Wang S, Voisin MB, Larbi KY, Dangerfield J, Scheiermann C, Tran M, Maxwell PH, Sorokin L, Nourshargh S. Venular basement membranes contain specific matrix protein low expression regions that act as exit points for emigrating neutrophils. J Exp Med. 2006;203:1519–1532. doi: 10.1084/jem.20051210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Watari K, Shibata T, Kawahara A, Sata K, Nabeshima H, Shinoda A, Abe H, Azuma K, Murakami Y, Izumi H, Takahashi T, Kage M, Kuwano M, Ono M. Tumor-derived interleukin-1 promotes lymphangiogenesis and lymph node metastasis through M2-type macrophages. PLoS One. 2014;9:e99568. doi: 10.1371/journal.pone.0099568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Weihrauch D, Zimmermann R, Arras M, Schaper J. Expression of extracellular matrix proteins and the role of fibroblasts and macrophages in repair processes in ischemic porcine myocardium. Cell Mol Biol Res. 1994;40:105–116. [PubMed] [Google Scholar]
- 296.Weis SM, Cheresh DA. Tumor angiogenesis: molecular pathways and therapeutic targets. Nat Med. 2011;17:1359–1370. doi: 10.1038/nm.2537. [DOI] [PubMed] [Google Scholar]
- 297.Weninger W, Biro M, Jain R. Leukocyte migration in the interstitial space of non-lymphoid organs. Nat Rev Immunol. 2014;14:232–246. doi: 10.1038/nri3641. [DOI] [PubMed] [Google Scholar]
- 298.Wigle JT, Harvey N, Detmar M, Lagutina I, Grosveld G, Gunn MD, Jackson DG, Oliver G. An essential role for Prox1 in the induction of the lymphatic endothelial cell phenotype. The EMBO journal. 2002;21:1505–1513. doi: 10.1093/emboj/21.7.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Wigle JT, Oliver G. Prox1 function is required for the development of the murine lymphatic system. Cell. 1999;98:769–778. doi: 10.1016/s0092-8674(00)81511-1. [DOI] [PubMed] [Google Scholar]
- 300.Willenborg S, Lucas T, van Loo G, Knipper JA, Krieg T, Haase I, Brachvogel B, Hammerschmidt M, Nagy A, Ferrara N, Pasparakis M, Eming SA. CCR2 recruits an inflammatory macrophage subpopulation critical for angiogenesis in tissue repair. Blood. 2012;120:613–625. doi: 10.1182/blood-2012-01-403386. [DOI] [PubMed] [Google Scholar]
- 301.Wu WK, Llewellyn OP, Bates DO, Nicholson LB, Dick AD. IL-10 regulation of macrophage VEGF production is dependent on macrophage polarisation and hypoxia. Immunobiology. 2010;215:796–803. doi: 10.1016/j.imbio.2010.05.025. [DOI] [PubMed] [Google Scholar]
- 302.Yang H, Kim C, Kim MJ, Schwendener RA, Alitalo K, Heston W, Kim I, Kim WJ, Koh GY. Soluble vascular endothelial growth factor receptor-3 suppresses lymphangiogenesis and lymphatic metastasis in bladder cancer. Mol Cancer. 2011;10:36. doi: 10.1186/1476-4598-10-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Yang J, Zhang L, Yu C, Yang X-F, Wang H. Monocyte and macrophage differentiation: circulation inflammatory monocyte as biomarker for inflammatory diseases. Biomarker Research. 2014;2:1–1. doi: 10.1186/2050-7771-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Ye D, Zhao Y, Hildebrand RB, Singaraja RR, Hayden MR, Van Berkel TJC, Van Eck M. The Dynamics of Macrophage Infiltration into the Arterial Wall during Atherosclerotic Lesion Development in Low-Density Lipoprotein Receptor Knockout Mice. The American Journal of Pathology. 2011;178:413–422. doi: 10.1016/j.ajpath.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Yona S, Kim KW, Wolf Y, Mildner A, Varol D, Breker M, Strauss-Ayali D, Viukov S, Guilliams M, Misharin A, Hume DA, Perlman H, Malissen B, Zelzer E, Jung S. Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis. Immunity. 2013;38:79–91. doi: 10.1016/j.immuni.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Yoshiji H, Kuriyama S, Kawata M, Yoshii J, Ikenaka Y, Noguchi R, Nakatani T, Tsujinoue H, Fukui H. The Angiotensin-I-converting Enzyme Inhibitor Perindopril Suppresses Tumor Growth and Angiogenesis: Possible Role of the Vascular Endothelial Growth Factor. Clinical Cancer Research. 2001;7:1073–1078. [PubMed] [Google Scholar]
- 307.Yotsumoto F, You WK, Cejudo-Martin P, Kucharova K, Sakimura K, Stallcup WB. NG2 proteoglycan-dependent recruitment of tumor macrophages promotes pericyte-endothelial cell interactions required for brain tumor vascularization. Oncoimmunology. 2015;4:e1001204. doi: 10.1080/2162402X.2014.1001204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Zajac E, Schweighofer B, Kupriyanova TA, Juncker-Jensen A, Minder P, Quigley JP, Deryugina EI. Angiogenic capacity of M1- and M2-polarized macrophages is determined by the levels of TIMP-1 complexed with their secreted proMMP-9. Blood. 2013;122:4054–4067. doi: 10.1182/blood-2013-05-501494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Zakrzewska A, Cui C, Stockhammer OW, Benard EL, Spaink HP, Meijer AH. Macrophage-specific gene functions in Spi1-directed innate immunity. Blood. 2010;116:e1–11. doi: 10.1182/blood-2010-01-262873. [DOI] [PubMed] [Google Scholar]
- 310.Zape JP, Zovein AC. Hemogenic endothelium: origins, regulation, and implications for vascular biology. Semin Cell Dev Biol. 2011;22:1036–1047. doi: 10.1016/j.semcdb.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 311.Zhang HH, Song K, Rabin RL, Hill BJ, Perfetto SP, Roederer M, Douek DC, Siegel RM, Farber JM. CCR2 identifies a stable population of human effector memory CD4+ T cells equipped for rapid recall response. J Immunol. 2010;185:6646–6663. doi: 10.4049/jimmunol.0904156. [DOI] [PubMed] [Google Scholar]
- 312.Zhang QW, Liu L, Gong CY, Shi HS, Zeng YH, Wang XZ, Zhao YW, Wei YQ. Prognostic significance of tumor-associated macrophages in solid tumor: a meta-analysis of the literature. PLoS One. 2012;7:e50946. doi: 10.1371/journal.pone.0050946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Zhao J, Chen L, Shu B, Tang J, Zhang L, Xie J, Qi S, Xu Y. Granulocyte/macrophage colony-stimulating factor influences angiogenesis by regulating the coordinated expression of VEGF and the Ang/Tie system. PLoS One. 2014;9:e92691. doi: 10.1371/journal.pone.0092691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Zheng W, Nurmi H, Appak S, Sabine A, Bovay E, Korhonen EA, Orsenigo F, Lohela M, D’Amico G, Holopainen T, Leow CC, Dejana E, Petrova TV, Augustin HG, Alitalo K. Angiopoietin 2 regulates the transformation and integrity of lymphatic endothelial cell junctions. Genes & development. 2014;28:1592–1603. doi: 10.1101/gad.237677.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Zhu Y, Knolhoff BL, Meyer MA, Nywening TM, West BL, Luo J, Wang-Gillam A, Goedegebuure SP, Linehan DC, DeNardo DG. CSF1/CSF1R blockade reprograms tumor-infiltrating macrophages and improves response to T-cell checkpoint immunotherapy in pancreatic cancer models. Cancer Res. 2014;74:5057–5069. doi: 10.1158/0008-5472.CAN-13-3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Ziegler-Heitbrock L, Hofer TP. Toward a refined definition of monocyte subsets. Front Immunol. 2013;4:23. doi: 10.3389/fimmu.2013.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Zumsteg A, Baeriswyl V, Imaizumi N, Schwendener R, Ruegg C, Christofori G. Myeloid cells contribute to tumor lymphangiogenesis. PLoS One. 2009;4:e7067. doi: 10.1371/journal.pone.0007067. [DOI] [PMC free article] [PubMed] [Google Scholar]