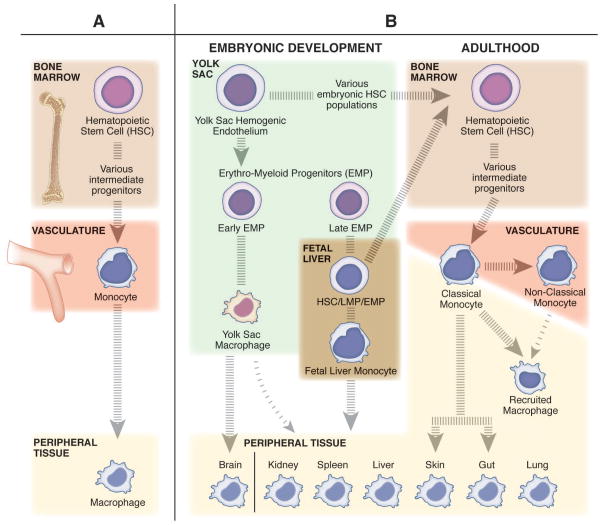
Figure 2. The diverse origins of macrophages and monocytes.
(A) The classic belief that tissue resident macrophages are populated from monocytes through adult hematopoiesis. In the bone marrow, hematopoietic stem cells (HSCs) differentiate into monocytes through a series of intermediate progenitors, extravasate from the vasculature, and differentiate into macrophages to maintain the tissue resident niches. (B) The more contemporary view that tissue resident macrophages undergo self-renewal, and have a distinct embryonic origin sourced from populations of erythro-myeloid progenitors (EMPs) derived from the yolk sac hemogenic endothelium. The early population of EMPs differentiate into yolk sac macrophages and then migrate to the tissues and contribute to the tissue resident macrophage population. The late population of EMPs gives rise to fetal liver monocytes through an unknown intermediate embryonic progenitor cell type (maybe EMP, HSC, or lympho-myeloid progenitor (LMP)). Fetal liver monocytes then go on to seed the tissue resident macrophage populations for all peripheral tissues other than the brain. Only the skin and gut resident macrophage populations are recognized to be replenished in homeostatic conditions by bone marrow derived cells, specifically classical monocytes. Classical monocytes (inflammatory monocytes) patrol the peripheral tissues in homeostatic conditions, while nonclassical (anti-inflammatory monocytes) surveil the vasculature. In inflammatory conditions, classical monocytes, and to a lesser extent nonclassical monocytes, can leave the vasculature and differentiate into recruited macrophages to transiently regulate the immune response.