Abstract
Background
Xue-Fu-Zhu-Yu decoction (XFZYD), Tian-Ma-Gou-Teng-Yin (TMGTY) and Wen-Dan decoction (WDD) are Chinese herbal formulas used to treat hypertension and cardiovascular diseases in traditional Chinese medicine (TCM). The goal of our study is to determine if XFZYD, TMGTY or WDD treatment ameliorated myocardial fibrosis in spontaneously hypertensive rats (SHRs) and to identify the mechanisms underlying any beneficial effects observed during the courses of the investigation.
Methods
Forty-five 12-week-old male spontaneously hypertensive rats and five age-matched male Wistar-Kyoto control rats were studied for 16 weeks. Each day 6 g∙kg−1 or 12 g∙kg−1 of XFZYD, TMGTY or WDD was orally administered at the indicated dose, and the systolic blood pressure (SBP) of all rats was measured using the tail-cuff method. Collagen levels were measured via hydroxyproline content assays and histological examination. Transforming growth factor beta-1 (TGF-β1) protein levels were determined via immunhistochemical and Western blot analysis. TGF-β1 mRNA levels were assessed using real-time reverse transcription polymerase chain reaction.
Results
Systolic blood pressure was unaffected, but collagen and TGF-β1 levels in SHRs treated with captopril and XFZYD (12 g∙kg−1) were significantly reduced when compared with untreated control SHRs. Administration of 12 g∙kg−1 XFZYD increased myocardial cell protection and decreased TGF-β1 mRNA and protein expression when compared with the other SHR treatment groups.
Conclusions
XFZYD treatment demonstrated a superior ability to reverse myocardial fibrosis when compared with WDD or TMGTY treatment in SHRs. XFZYD also decreased TGF-β1 mRNA and protein expression, suggesting that the TGF-β1 signaling pathway plays a role in the therapeutic effects of XFZYD treatment.
Electronic supplementary material
The online version of this article (doi:10.1186/s12906-016-1030-3) contains supplementary material, which is available to authorized users.
Keywords: Xue-Fu-Zhu-Yu decoction, Tian-Ma-Gou-Teng-Yin, Wen-Dan decoction, Hypertension, Myocardial fibrosis, SHR, TGF-β1
Background
Hypertension is one of the most common diseases afflicting humans worldwide. It is a major risk factor for stroke, myocardial infarction, vascular disease and chronic kidney disease. Hypertension may contribute to heart failure in as many as 50 to 60 % of patients. It contributes to 50 % of ischemic strokes and increases the risk of hemorrhagic stroke. Hypertension is the primary cause for myocardial fibrosis, and contributes to the pathophysiology of cardiac damage [1, 2]. Hypertension causes a sustained increase in cardiac pressure overload, triggering excessive proliferation and the accumulation of interstitial and perivascular collagen fibers that leads to myocardial fibrosis [3]. Myocardial fibrosis is an integral component of chronic heart disease [4], and it is increasingly thought to have a key role in disease progression as well [5]. Unfortunately, no specific therapies capable of halting or reversing myocardial fibrosis are currently available. Therefore, myocardial fibrosis prevention and treatment is a subject of great clinical value and public health interest.
Transforming growth factor β1 (TGF-β1) is a key regulatory factor involved in the proliferation and differentiation of fibroblasts [6]. Recent pharmacological studies targeting TGF-β1 have reported progress in the development of myocardial fibrosis treatments [7], but the clinical efficacy of these treatments has yet to be established.
Traditional Chinese medicine (TCM) employs mixtures of multiple herbs to treat patients. These mixtures are referred to as formulas or “Fufang”. It is currently thought that the therapeutic effects that have been demonstrated for many specific TCM therapies are mediated by multiple ingredients. Pharmacologically active ingredients have been demonstrated to be present in several of these formulas, and some of these formulas have been reported to be therapeutically effective against multiple diseases, including tumors, inflammatory conditions and cardiac diseases [8].
Spontaneously hypertensive rat (SHR) is a well-established model for studying the mechanisms of hypertrophy and heart failure associated with genetic hypertension. SHR is often used as a model of human essential hypertension-induced myocardial fibrosis [9]. The SHR develops hypertension at 2 to 6 weeks, followed by progressive myocardial fibrosis, cardiac hypertrophy, and eventual heart failure [10, 11]. Typical symptoms of myocardial fibrosis include left ventricular hypertrophy (LVH) and age-related collagen increase [12]. Several groups reported myocardial collagen concentration and elevated collagen synthesis in SHR when compared with WKY rats of similar age [13, 14]. The myocardial fibrosis and hypertrophy in SHR can be prevented using specific antihypertensive agents [15].
Numerous studies published in the Chinese medical literature have reported the successful treatment of symptoms related to high blood pressure, such as headaches and dizziness, using the herbal formulas Xue-Fu-Zhu-Yu decoction (XFZYD), Tian-Ma-Gou-Teng-Yin (TMGTY) and Wen-Dan decoction (WDD) [16, 17]. Previous studies showed that the three Chinese formulas have significant effects on a variety of hypertension caused cardiovascular diseases. For example, TMGTY can counteract myocardial fibrosis in SHR by reducing LVI and collagen content [18]. Otherwise, the effect of TMGTY on arterial pressure was through an action of TMGTY on sympathetic vasomotor activity [19]. XFZYD improves endothelial function and the state before hypertensive thrombosis in the vessels of the SHRs with hypertension and reduces the formation of myocardial collagenIand III [20]. XFZYD could also improve the ischemic necrosis and promoting the angiogenesis [21]. WDD effectively regulates the lipid metabolism, prevent and treat the hyperlipidemia induced diseases in SHR [22]. Therefore, we assume that three Chinese formulas have effect on reversion of hypertensive myocardial fibrosis in SHR. However, the underlying mechanisms of action and therapeutic effects of treatment with these herbal formulas remain unclear.
The present study aimed to investigate whether XFZYD, TMGTY or WDD were capable of reversing myocardial fibrosis in SHR and to elucidate the mechanisms underlying any therapeutic effects found.
Methods
Drug preparation
Traditional Chinese herb granules manufactured by Guangdong EFONG Pharmaceutical Co. Ltd. (Guangzhou, China) were purchased from the pharmacy of Southern Hospital. Captopril tablets (12.5 mg) were purchased from Bristol-Myers Squibb Co. Ltd. (Shanghai, China; Lot number: 1209031). The components comprising the TMGTY, WDD and XFZYD formulas are listed in Table 1, and are based on established quality control procedures [8, 19, 23]. After preparation, the herbal mixtures were freeze-dried. Prior to use, each 2 g of dry herbal mixture was resuspended in 1 ml distilled water.
Table 1.
Composition of Chinese herbal formulas
| Components | Source | Amount used (g) | Lot number |
|---|---|---|---|
| Tian-Ma-Gou-Teng-Yin (TMGTY) | |||
| Gastrodia elata Blume | Root | 9 | 407233 T |
| Uncaria rhynchophylla (Miq.) Miq. ExHavil. | Hook | 12 | 404145 T |
| Reynoutria multiflora (Thunb.) Moldenke | Aerial part | 9 | 404203 T |
| Haliotis diversicolor Reeve | Conch | 18 | 311190 T |
| Eucommia ulmoides Oliv. | Cortex | 9 | 407270 T |
| Scutellaria baicalensis Georgi | Root | 9 | 409032 T |
| Gardenia jasminoides J.Ellis | Fruit | 9 | 407018 T |
| Leonurus japonicas Houtt. | Aerial part | 9 | 409112 T |
| Viscum coloratum (Kom.) Nakai | Stem | 9 | 408443 T |
| Achyranthes bidentata Blume | Root | 12 | 409081 T |
| Poria cocos (Schw.) Wolf | Sclerotium | 9 | 406029 T |
| Wen-Dan decoction (WDD) | |||
| Pinellia ternata (Thunb.) Makino | Tuber | 9 | 408101 T |
| Citrus × aurantium L. | Young fruit | 12 | 401084 T |
| Citrus reticulata Blanco | Mature pericarp | 9 | 409424 T |
| Pleioblastus amarus (Keng) Keng f. | Stem | 9 | 407209 T |
| Glycyrrhiz auralensis Fisch. | Rhizome | 5 | 408447 T |
| Poria cocos (Schw.) Wolf | Sclerotium | 5 | 408407 T |
| Xue-Fu-Zhu-Yu decoction (XFZYD) | |||
| Angelica sinensis (Oliv.) Diels | Root | 9 | 410141 T |
| Bupleurum chinense DC. | Root | 3 | 407027 T |
| Carthamus tinctorius L. | Flower | 9 | 408165 T |
| Citrus aurantium L. | Fruit | 6 | 403011 T |
| Cyathula officinalis Kuan | Root | 9 | 409101 T |
| Glycyrrhiza glabra L. | Root | 3 | 408447 T |
| Ligusticum striatum DC. | Root | 5 | 410141 T |
| Paeonia lactiflora Pall. | Root | 6 | 407380 T |
| Platycodon grandiflorus (Jacq.) A. DC. | Root | 5 | 408059 T |
| Prunus persica (L.) Batsch. | Seed | 12 | 406423 T |
| Rehmannia glutinosa (Gaertn.) DC. | Root | 9 | 403409 T |
Animal studies
Forty-five 12-wk-old male SHRs (280 – 320 g) and five age-matched male Wistar-Kyoto (WKY) rats (285–310 g) were purchased from VITAL RIVER Co. Ltd. (Beijing, China; certificate number, SCXK2012-001). SHRs were selected based on total hypertension and established myocardial fibrosis. The WKY rats were used as controls. All rats were maintained in plastic cages with soft bedding under a 12/12 h light–dark cycle. The rats received standard care and had free access to a standard diet and drinking water. The protocol of this study was reviewed and approved by the Animal Review Board of Southern Medical University (Guangzhou, China). The research was conducted under the Animal [Scientific Procedures] Act of 1986 and the institutional guidelines of Southern Medical University for the care and use of animals.
The SHRs were randomized into nine groups with five rats in each group. The group 1 rats were treated with distilled water and served as the SHR no-treatment control group. Groups 2 and 3 were treated with 6 g∙kg−1 and 12 g∙kg−1 TMGTY, respectively. Groups 4 and 5 were treated with 6 g∙kg−1 and 12 g∙kg−1 WDD, respectively. Groups 6 and 7 were treated with 6 g∙kg−1 and 12 g∙kg−1 XFZYD, respectively. Groups 8 and 9 were treated with 30 mg∙kg−1 and 60 mg∙kg−1 captopril, respectively, and were used as the positive treatment control group. Each SHR was given daily oral administration of the appropriate designated drug. The WKY control rats were also treated with distilled water as similar with the group 1 rats for the blank control. All the rats were fed under Specific Pathogen-Free (SPF) conditions, with fresh, sterilized food, vegetables and water that were changed each day. Bedding was replaced once every two days. Environmental noise was less than 60 dB, temperature was maintained between 23 and 26 °C and humidity was maintained between 40 and 70 %.
The total treatment course was 16 weeks. Rat body weight was monitored each wk and systolic blood pressure was measured before and after each experiment. All rats survived the study, and no significant abnormalities were observed. After the final treatment, rats were anesthetized using 350 mg/kg of 10 % chloral hydrate and then euthanized using cervical dislocation. The heart was quickly removed from each euthanized rat, washed with cold normal saline and then dried with filter paper. One-hundred mg of the left ventricle was removed and stored at −70 °C for further analysis. The remainder of the left ventricle was fixed in 10 % formalin for histology.
Systolic blood pressure measurements
Rats were housed in a 37 °C chamber for 10 min for indirect systolic blood pressure measurements, then transferred to a standard blood pressure measurement apparatus that included a heating pad, acrylic restrainer, tail cuff and pulse sensor (NarcoBiosystems, Houston, TX, USA). The tail cuff was connected to a cylinder of compressed air via inlet and outlet valves that allowed steady inflation and deflation of the cuff. Tail cuff pressure was continuously recorded using a solid-state pressure sensor (Sensym, Honeywell Sensing & Control, Inc., Morristown, NJ, USA). Pulse and pressure sensor signals were amplified and then digitized with an analogy-digital board (DT16EZ, Data Translation, Inc., Marlboro, MA, USA) connected to a desktop computer. Labtech Notebook Pro (Laboratory Technology Corp., Wilmington, MA, USA) was used to control and display the procedure online. Inflation and deflation scores and the compression interval were recorded for each rat [24].
Hydroxyproline content assay
The chloramine T assay was used to quantify hydroxyproline (HYP) in the tissues collected from the rats in this study [25]. The apex of the left ventricle of each rat was defatted and lysed. Next, the sample was centrifuged at 4000 rpm for 10 min. After centrifugation, the supernatant was mixed with fresh chloramine T for 10 min, followed by mixture with Ehrlich’s reagent at 75 °C for 20 min. Samples were then cooled, and the optical density of each sample was read at 560 nm with a microplate reader (iMark, Bio-Rad laboratories, USA). Prior to measuring each sample, the microplate reader was adjusted using a “blank” sample that was prepared using the same procedure used to prepare the experimental samples, but without any cardiac tissue in the reaction mixture. HYP concentration, expressed as micrograms per milligram of dry heart weight, was then calculated as previously described [26].
Histological examination
The remaining ventricular tissue not used in the hydroxyproline content assay was immediately fixed in 10 % formalin, embedded in paraffin, sliced into 5 μm sections and then heated for 2 h in a 65 °C incubator. These sections were stained using the Masson trichrome method to examine myocardial interstitial fibrosis [27]. Each section was photographed at 400 x magnification using a light microscope (CX31, Olympus, Tokyo, Japan). All images were analyzed using Image-pro Plus 6.0 software (Media Cybernetics, Bethesda, MD, USA).
The percentage of collagen present in each microscopic field represented the myocardial interstitial collagen content. The accumulation of collagen content in the interstitial spaces of the left ventricle were assessed using polarized light microscopy and analyzed using Image-pro Plus 6.0 software.
Immunohistochemical analysis of TGF-β1 expression
The middle ring of the left ventricle was fixed in 10 % neutral formalin for 24 h, embedded in paraffin and cut into 4 μm sections. Each section was incubated at 4 °C overnight with anti-TGF-β1 antibody (1:1000, Proteintech Co., Chicago, IL, USA) and at 37 °C for 30 min with Mo/Rb type I polymer (Genecompany, Guangzhou, China). The streptavidin-biotin-peroxidase complex technique (Histostain Plus Kit, cat. No. 85–8943; Zymed, Life Technologies, Grand Island, NY, USA) was used for immunohistochemical visualization of antibody-bound sections. Sections were observed at 400 x magnification, and TGF-β1 staining intensity was analyzed using Image-pro Plus 6.0 software. Twenty-five random fields per section were analyzed and combined to obtain a final value for each section.
TGF-β1 mRNA expression measurement
TGF-β1 mRNA expression was determined using real-time reverse transcription polymerase chain reaction (RT-PCR). Total RNA was extracted from tissues using Trizol (Sigma, St Louis, MO, USA). RNA yields and purity were assessed by spectrophotometric analysis (BioPhotometer plus, Eppendorf, Germany). Total RNA (1 μg/μl) transcription was performed using an in vitro transcription Kit (PrimeScript RT reagent Kit Perfect Real Time, TaKaRa, Japan). RT-PCR reactions were performed with 20 μl reactions that consisted of 10 μl 2X SYBR Premix ExTaq, 0.4 μl PCR Forward primer (10 μM), 0.4 μl PCR reverse primer (10 μM), 2 μl cDNA, 0.4 μl ROX Reference Dye II and 6.8 μl double-distilled water. PCR was conducted with an initial denaturation step of 95 °C for 30 s, followed by 40 cycles of denaturation at 95 °C for 5 s and 60 °C annealing for 34 s. All RT-PCR reactions were performed in triplicate for each sample. The primer sequences were as follows:
TGF-β1
Forward: 5’ - CATTGCTGTCCCGTGCAGA - 3’
Reverse: 5’ - AGGTAACGCCAGGAATTGTTGCTA - 3’
β-actin
Forward: 5’ - TGACGTTGACATCCGTAAAGACC - 3’
Reverse: 5’ - GTGCTAGGAGCCAGGGCAGTAA - 3’
RT-PCR and data analysis were carried out using ABI 7500 Software 2.0.3 (Life Technologies). Values obtained for TGF-β1 were normalized against values obtained for β-actin, and the results were expressed as relative integrated intensity.
Western blot analysis of TGF-β1
Heart tissue was homogenized in ice-cold loading buffer (pH 6.8). Total protein was extracted using RIPA Lysis Buffer (Fu De Biological Technology, Hangzhou, China). The protein was separated using a 12 % SDS-polyacrylamide gel in electrophoresis sample buffer and then transferred to PVDF membranes. After blocking membranes for 1.5 h with 5 % bovine serum albumin (BSA) in tris-buffered saline containing 0.1 % Tween-20 at pH 7.6 (TBST), the membranes were probed overnight at 4 °C with antibodies against TGF-β1 (1:1500, product number: ab92486, Epitomics, Burlingame, California, USA and Abcam, Cambridge, UK). After the overnight primary antibody incubation, the membranes were washed with TBST, once for 15 min and then an additional three times for 5 min each. After washing, the membranes were incubated with goat anti-rabbit horseradish peroxidase-conjugated secondary antibody (1:6000, product number: #7074, Cell Signaling Technology, Danvers, MA, USA) for 1 h at room temperature and then washed three times with TBST for a total of 30 min. Antibody-antigen complexes were visualized using enhanced chemiluminescence (Millipore, USA). Images were scanned and the results were quantified using Image-pro Plus 6.0 software.
Statistical analysis
All data were presented as means ± standard deviation (SD). Multiple comparisons were performed using one-way ANOVA (p < 0.05) followed by Dunnett’s post-hoc t-test. All statistical analyses were performed using SPSS 13.0 software (SPSS Inc. Chicago, IL, USA).
Results
Effects of TCM on systolic blood pressure and body weight
Data obtained during this study were consistent with data reported by previous studies, and indicated that the systolic blood pressure of control SHRs was much higher than that of WKY rats (Table 2, p = 0.005). Comparisons among the SHRs treated with WDD, XFZYD, TMGTY and captopril revealed that captopril treatment alone significantly reduced systolic blood pressure (p = 0.032). None of the other treatments demonstrated any anti-hypertensive effect (p = 0.085, WDD; p = 0.075, XFZYD; p = 0.103, TMGTY).
Table 2.
Effects of TCM on systolic blood pressure and body weight
| SHRs | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| WKY | Control | TMGTY | WDD | XFZYD | CAPTOPRIL | |||||
| 6 g∙kg−1 | 12 g∙kg−1 | 6 g∙kg−1 | 12 g∙kg−1 | 6 g∙kg−1 | 12 g∙kg−1 | 30 mg∙kg−1 | 60 mg∙kg−1 | |||
| Systolic blood pressure (mmHg) | ||||||||||
| Before | 123 ± 13 | 177 ± 8** | 173 ± 13 | 176 ± 9 | 175 ± 11 | 179 ± 7 | 172 ± 14 | 170 ± 16 | 177 ± 13 | 178 ± 11 |
| After | 128 ± 11 | 188 ± 6** | 179 ± 8 | 178 ± 6 | 177 ± 7 | 180 ± 9 | 170 ± 12 | 168 ± 5 | 161 ± 10* *** | 153 ± 9* *** |
| Body weight (g) | ||||||||||
| Before | 299 ± 7 | 310 ± 5 | 305 ± 9 | 303 ± 6 | 302 ± 7 | 306 ± 7 | 294 ± 11 | 298 ± 8 | 305 ± 7 | 302 ± 10 |
| After | 361 ± 12 | 363 ± 4 | 345 ± 10 | 351 ± 11 | 342 ± 13 | 336 ± 5 | 332 ± 10 | 330 ± 9 | 330 ± 8 | 329 ± 14 |
**p < 0.01 compared to the WKY rats, ***p < 0.05 compared to the SHR control
*p < 0.05 compared to the corresponding data before the experiment
Effect of TCM on myocardial HYP content
Myocardial HYP content levels are illustrated in Fig. 1. TMGTY, WDD, XFZYD and captopril treatment all significantly reduced the left ventricular HYP content of SHRs. The strongest effect was seen after captopril (p < 0.001, F = 94.431, df = 17, low-dose group; p = 0.003, F = 110.054, df = 17, high-dose group) and XFZYD (p < 0.001, low- and high-dose groups) treatment. High dose treatment groups exhibited a stronger HYP reduction than the low dose treatment groups.
Fig. 1.
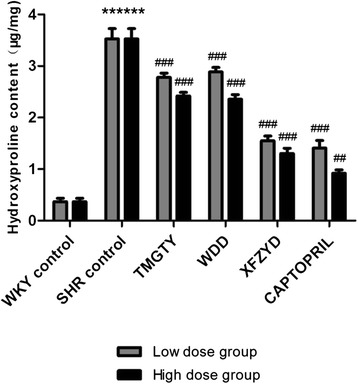
Hydroxyproline levels in the left ventricle in SHR and WKY (n = 3)
Effect of TCM on myocardial collagen
Red or deep red normal muscle fibers and blue myocardial interstitial collagen fibers were observed during microscopic examination of Masson trichrome stained tissues (Fig. 2). Generally, less collagen accumulation was seen in the myocardia of WKY tissues when compared with tissues from the SHR control group (p = 0.041; F = 74.927, df = 17; Fig. 2). After treatment, collagen levels were significantly reduced in all treatment groups. Notably, less collagen was deposited in the SHR groups treated with the TCM formulas when compared with the collagen levels of the control SHR group, and both XFZYD (12 g∙kg−1, p = 0.018) and captopril (60 mg∙kg−1, p = 0.007) significantly reduced the post-treatment myocardial collagen levels. The observed reduction in myocardial collagen was not statistically significant after treatment with TMGTY (12 g∙kg−1, p = 0.064) or WDD (12 g∙kg−1, p = 0.092).
Fig. 2.
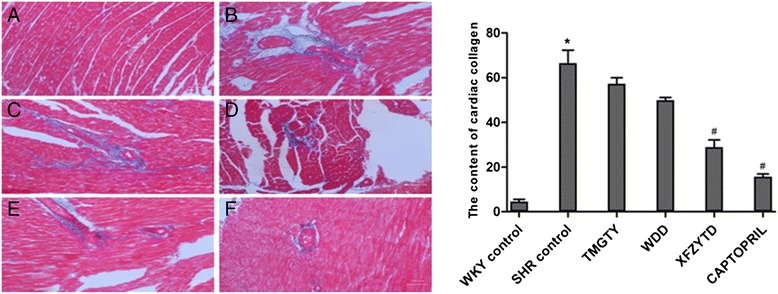
Representative micrographs of cardiac collagen in the interstitial space of the left ventricle. (n = 3) (a) WKY control, (b) SHR control, (c) SHR with 12 g∙kg−1 TMGTY, (d) SHR with 12 g∙kg−1 WDD, (e) SHR with 12 g∙kg−1 XFZYD and (f) SHR with 60 mg∙kg−1 captopril
Effect of TCM on myocardial TGF-β1
TGF-β1 expression in the myocardial interstitium of SHR controls was significantly increased when compared with the myocardial interstitium of WKY control rats (p = 0.039; F = 73.108, df = 17; Fig. 3). Immunohistochemistry revealed that the captopril (60 mg∙kg−1, p = 0.021) and XFZYD (12 g∙kg−1, p = 0.034) treated groups exhibited significantly reduced brown granule deposits, suggesting that TGF-β1 expression was reduced in these groups when compared with the control SHR group (Table 3). The concentrations of brown granule deposits were not significantly different between the TMGTY (12 g∙kg−1, p = 0.071) and WDD (12 g∙kg−1, p = 0.078) treatment groups.
Fig. 3.
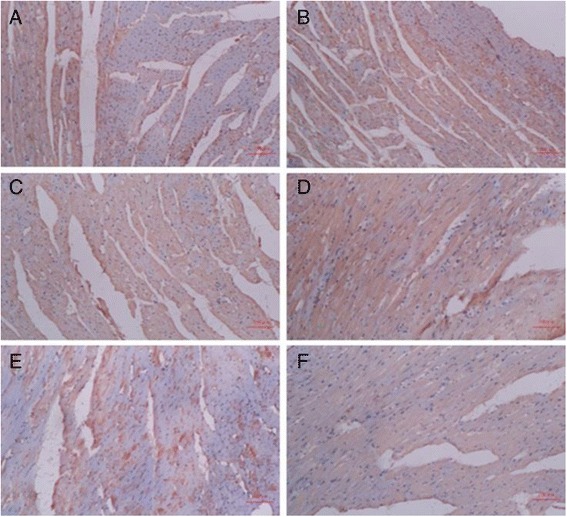
Representative micrographs of TGF-β1 protein expression in myocardial tissue. (n = 3) (a) WKY control, (b) SHR control, (c) SHR with 12 g∙kg−1 TMGTY, (d) SHR with 12 g∙kg−1 WDD, (e) SHR with 12 g∙kg−1 XFZYD and (f) SHR with 60 mg∙kg−1 captopril
Table 3.
Effects of TCM on myocardial TGF-β1
| Sample | IOD | Area(μm2) | Density |
|---|---|---|---|
| WKY control | 1839 ± 75 | 2290 ± 122 | 0.81 ± 0.07 |
| SHR control | 1225 ± 39 | 1018 ± 20 | 1.20 ± 0.01* |
| TMGTY | 1306 ± 74 | 1179 ± 58 | 1.12 ± 0.12 |
| WDD | 1740 ± 46 | 1580 ± 85 | 1.11 ± 0.08 |
| XFZYTD | 921 ± 43 | 1009 ± 43 | 0.92 ± 0.09** |
| CAPTOPRIL | 591 ± 60 | 708 ± 11 | 0.83 ± 0.07** |
*p < 0.05 compared to the WKY control, **p < 0.05 compared to the SHR control
TGF-β1 mRNA expression in SHR and WKY
TGF-β1 mRNA expression was increased in the left ventricles of control SHRs when compared with WKY control rats (p = 0.007; F = 152.538, df = 17, low-dose group; F = 137.812, df = 17, high-dose group; Fig. 4). Treatment with captopril (30 mg∙kg−1, p = 0.025; 60 mg∙kg−1, p = 0.009) and XFZYD (12 g∙kg−1, p = 0.031) significantly reduced TGF-β1 mRNA expression in a dose-dependent manner. In contrast, neither TMGTY (6 g∙kg−1, p = 0.095; 12 g∙kg−1, p = 0.076) nor WDD (6 g∙kg−1, p = 0.101; 12 g∙kg−1, p = 0.078) treatment had any effect on TGF-β1 mRNA expression.
Fig. 4.
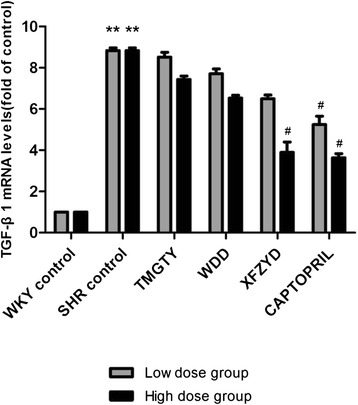
TGF-β1 mRNA expression in SHR and WKY. (n = 3)
TGF-β1 protein expression in SHR and WKY
TGF-β1 protein expression differed significantly between the control SHR group and the WKY control group (p < 0.001; F = 1408.471, df = 17; Fig. 5). Furthermore, TGF-β1 protein expression in SHRs was significantly decreased in the captopril (60 mg∙kg−1) and XFZYD (12 g∙kg−1) treatment groups when compared with the control SHR group (p < 0.001). Treatment with WDD (12 g∙kg−1) also markedly reduced TGF-β1 protein expression when compared with the control SHR group (p = 0.036). In contrast, TMGTY (12 g∙kg−1) treatment was not associated with a statistically significant decrease in TGF-β1 protein expression (p = 0.064).
Fig. 5.
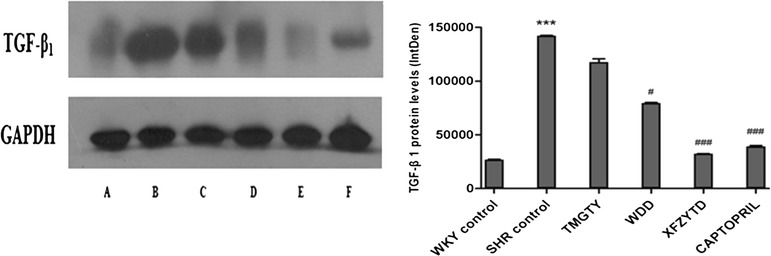
Representative TGF-β1 Western blots. (n = 3) (a) WKY control, (b) SHR control, (c) SHR with 12 g∙kg−1 TMGTY, (d) SHR with 12 g∙kg−1 WDD, (e) SHR with 12 g∙kg−1 XFZYD and (f) SHR with 60 mg∙kg−1 captopril
Discussion
Systemic hypertension is a highly prevalent chronic disease and a significant determinant of cardiovascular morbidity and mortality [28, 29]. It is a primary cause of myocardial fibrosis, which in turn plays a key role in the pathophysiology of hypertension-induced cardiac damage [30]. Myocardial fibrosis is the excessive deposition of collagen fibers in myocardial tissue [31], and has been closely associated with hypertension, myocardial infarction, atherosclerosis and diabetes. Thus, the inhibition of early hypertension may delay ventricular remodeling and heart failure [32].
Captopril is an angiotensin-converting enzyme inhibitor that is used as an anti-hypertensive agent. It is also ameliorates myocardial fibrosis [33], and is used to treat certain types of congestive heart failure [34]. In this study, we compared the efficacy of captopril with three different Chinese herbal formulas derived from TCM for the treatment of myocardial fibrosis in SHRs.
The baseline blood pressure measured in this study was much higher in SHRs when compared with the baseline blood pressure in WKY rats. WDD, XFZYD and TMGTY treatment had no effect on the systolic blood pressure, whereas captopril treatment significantly reduced the systolic blood pressure measured in SHRs. XFZYD treatment at 12 g∙kg−1 each day for 16 weeks effectively inhibited myocardial fibrosis and decreased HYP in SHR myocardial tissue. Our results suggest that the XFZYD induced reduction in fibrosis and HYP content was independent of blood pressure, providing a theoretical basis for the clinical application of XFZYD.
Cardiac hypertrophy and interstitial fibrosis are common outcomes after cardiac injury and overload [35, 36]. TGF-β overexpression is a clear factor underlying tissue fibrosis development in numerous human diseases. Furthermore, TGF-β plays a crucial role in pathogenic processes associated with cardiac remodeling and fibrosis [37], and the over expression of cardiac TGF-β1 is associated with both fibrosis and hypertrophy [38].
Using immunohistochemical, RT-PCR and Western blot analysis, we confirmed that the expression of TGF-β1 mRNA and protein was reduced in SHRs after 16 weeks of daily treatment with 12 g∙kg−1 XFZYD. We conclude that XFZYD inhibited fibrosis by disrupting the TGF-β signaling pathway. Other TCM treatments had no significant effects.
XFZYD is a classic formula in TCM clinical for treatment of heart disease, our study found that XFZYD has the effect of reverse hypertensive myocardial fibrosis and the mechanism may be associated with TGF-β1 signaling pathways. So we speculate that there are some active components in the formula of XFZYD to ameliorate hypertensive myocardial fibrosis through TGF-β1 signaling pathways. Studies show that XFZYD not only can inhibit myocardial fibroblasts proliferation, but also to disturb the action of collagen secretion by myocardial fibroblasts [39]. Ferulic acid sodium is the main active ingredient in Angelica sinensis (Oliv.) Diels, the structure contains phenolic hydroxyl, which has the function of antioxidant and antiatherosclerosis. Ferulic acid sodium can suppress the cardiac fibroblasts proliferation induced by AngII, which exhibit a dose-dependent effect, the mechanism may be associated with lower TGF-β1 protein expression [40]. Ligustrazine is the main component of Ligusticum striatum DC., which can block and delay the development of myocardial fibrosis in chronic pressure overload rats and also inhibit the myocardial fibroblasts induced by AngII [41]. Carthamin yellow is extracted from Carthamus tinctorius L., which can inhibit AngII and ACE and also play a good role in cardiovascular protection [42]. Amygdalin in Prunus persica (L.) Batsch has the effect of ameliorate hepatic fibrosis [43]. Paeoniflorin in Paeonia lactiflora Pall. has protective effect on myocardium in rats [44]. The existing research results are consistent with our conclusion, which can support each other. We can infer that the effective components of XFZYD formula to ameliorate myocardial fibrosis through TGF-β1 signaling pathways may have close relationship with ferulic acid sodium, ligustrazine, carthamin yellow, amygdalin and paeoniflorin which are extracted respectively from Angelica sinensis (Oliv.) Diels, Ligusticum striatum DC., Carthamus tinctorius L., Prunus persica (L.) Batsch and Paeonia lactiflora Pall.. We plans to research the demolition party of XFZYD in next step, and focus on the relationship between each active ingredient and TGF-β1 signaling pathway, in order to screen out the exact active ingredients to reverse hypertensive myocardial fibrosis in XFZYD.
Conclusion
Among the three TCM formulas tested in this study, we found that only XFZYD was capable of reversing myocardial fibrosis. We also found that XFZYD treatment decreased hypertension-induced cardiac fibrosis independent of systolic blood pressure. Future clinical studies are needed to evaluate the therapeutic benefit of XFZYD in patients with hypertension. This study demonstrates that XFZYD decreases cardiac fibrosis via TGF-β1 signaling pathway.
Additional files
The protocol of animal experimental. (DOCX 796 kb)
The approval of the Southern Medical University Animal Cave and Use Committee. (DOCX 1607 kb)
The constitution of the Southern Medical University Animal Cave and Use Committee. (DOCX 3226 kb)
The staff of the Southern Medical University Animal Cave and Use Committee. (DOCX 658 kb)
The HPLC results for the quality control of the herbs. (PDF 27 kb)
Acknowledgements
This study was supported by The National Natural Science Foundation, grant numbers 81072937 and 81373811 from the Science Fund Committee of China.
Abbreviations
- CVF
Collagen volume fraction
- SBP
Systolic blood pressure
- SHR
Spontaneously hypertensive rat
- TCM
Traditional Chinese Medicine
- TMGTY
Tian-Ma-Gou-Teng-Yin
- WDD
Wen-Dan decoction
- WKY
Wistar-Kyoto rats
- XFZYD
Xue-Fu-Zhu-Yu decoction
Footnotes
Guohua Zhang and Guang Yang are co-first author.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
LL and GZ designed the study. GZ and GY performed the experiments. XZ and WW analyzed data. LL and GY wrote the manuscript. JH, JR and YY revised the manuscript. All authors read and approved the final manuscript. GZ and GY contributed equally to this work and should be considered co-first authors.
Contributor Information
Guohua Zhang, Email: zghgz@163.com.
Guang Yang, Email: xshfh12315@163.com.
Yan Deng, Email: 362186548@qq.com.
Xiangling Zhao, Email: 408605446@qq.com.
Yingbao Yang, Email: yangyb@smu.edu.
Jinjun Rao, Email: raojj@smu.edu.cn.
Wenya Wang, Email: wangwy@fimmu.com.
Xin Liu, Email: 282412293@qq.com.
Jian He, Email: jianhe@smu.edu.cn.
Lin Lv, Phone: +08602061648536, Email: lynnlv1@163.com.
References
- 1.Ozawa K, Funabashi N, Kataoka A, Tanabe N, Yanagawa N, Tatsumi K, et al. Myocardial fibrosis in the right ventricle detected on ECG gated 320 slice CT showed a short term poor prognosis in subjects with pulmonary hypertension. Int J Cardiol. 2013;168(1):584–586. doi: 10.1016/j.ijcard.2013.01.251. [DOI] [PubMed] [Google Scholar]
- 2.Medvedev NV, Gorshukova NK. Pathogenetic significance of interstitial fibrosis in development myocardial dysfunction and chronic heart failure in elderly patients with arterial hypertension. Adv Gerontol. 2013;26(1):130–136. [PubMed] [Google Scholar]
- 3.Sowers JR. Hypertension myocardial fibrosis. J Clin Hypertens. 2007;9(7):558–559. doi: 10.1111/j.1524-6175.2007.07274.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xu X, Tan X, Tampe B, Nyamsuren G, Liu X, Maier LS, et al. Epigenetic balance of aberrant Rasal1 promoter methylation and hydroxymethylation regulates cardiac fibrosis. Cardiovasc Res. 2015;105(3):279–291. doi: 10.1093/cvr/cvv015. [DOI] [PubMed] [Google Scholar]
- 5.Khan R, Sheppard R. Fibrosis in heart disease: understanding the role of transforming growth factor-beta in cardiomyopathy, valvular disease and arrhythmia. Immunology. 2006;118(1):10–24. doi: 10.1111/j.1365-2567.2006.02336.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leask A. TGFbeta, cardiac fibroblasts, and the fibrotic response. Cardiovasc Res. 2007;74(2):207–212. doi: 10.1016/j.cardiores.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 7.Yoshimatsu Y, Watabe T. Roles of TGF-beta signals in endothelial-mesenchymal transition during cardiac fibrosis. Int J Inflamm. 2011;2011:724080. doi: 10.4061/2011/724080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee JJ, Hsu WH, Yen TL, Chang NC, Luo YJ, Hsiao G, et al. Traditional Chinese medicine, Xue-Fu-Zhu-Yu decoction, potentiates tissue plasminogen activator against thromboembolic stroke in rats. J Ethnopharmacol. 2011;134(3):824–830. doi: 10.1016/j.jep.2011.01.033. [DOI] [PubMed] [Google Scholar]
- 9.Ziegelhoffer-Mihalovicova B, Arnold N, Marx G, Tannapfel A, Zimmer HG, Rassler B. Effects of salt loading and various therapies on cardiac hypertrophy and fibrosis in young spontaneously hypertensive rats. Life Sci. 2006;79(9):838–846. doi: 10.1016/j.lfs.2006.02.041. [DOI] [PubMed] [Google Scholar]
- 10.Bing OH, Brooks WW, Robinson KG, Slawsky MT, Hayes JA, Litwin SE, et al. The spontaneously hypertensive rat as a model of the transition from compensated left ventricular hypertrophy to failure. J Mol Cell Cardiol. 1995;27(1):383–396. doi: 10.1016/S0022-2828(08)80035-1. [DOI] [PubMed] [Google Scholar]
- 11.Conrad CH, Brooks WW, Hayes JA, Sen S, Robinson KG, Bing OH. Myocardial fibrosis and stiffness with hypertrophy and heart failure in the spontaneously hypertensive rat. Circulation. 1995;91(1):161–170. doi: 10.1161/01.CIR.91.1.161. [DOI] [PubMed] [Google Scholar]
- 12.Engelmann GL, Vitullo JC, Gerrity RG. Morphometric analysis of cardiac hypertrophy during development, maturation, and senescence in spontaneously hypertensive rats. Circ Res. 1987;60(4):487–494. doi: 10.1161/01.RES.60.4.487. [DOI] [PubMed] [Google Scholar]
- 13.Cutilletta AF, Erinoff L, Heller A, Low J, Oparil S. Development of left ventricular hypertrophy in young spontaneously hypertensive rats after peripheral sympathectomy. Circ Res. 1977;40(4):428–434. doi: 10.1161/01.RES.40.4.428. [DOI] [PubMed] [Google Scholar]
- 14.Sen S, Bumpus FM. Collagen synthesis in development and reversal of cardiac hypertrophy in spontaneously hypertensive rats. Am J Cardiol. 1979;44(5):954–958. doi: 10.1016/0002-9149(79)90228-5. [DOI] [PubMed] [Google Scholar]
- 15.Sen S, Tarazi RC, Khairallah PA, Bumpus FM. Cardiac hypertrophy in spontaneously hypertensive rats. Circ Res. 1974;35(5):775–781. doi: 10.1161/01.RES.35.5.775. [DOI] [PubMed] [Google Scholar]
- 16.Xue M, Chen KJ, Ma XJ, Liu JG, Jiang YR, Miao Y, et al. Effects of Xuefu Zhuyu Oral Liquid on hemorheology in patients with blood-stasis syndrome due to coronary disease and their relationship with human platelet antigen-3 polymorphism. Zhong Xi Yi Jie He Xue Bao. 2008;6(11):1129–1135. doi: 10.3736/jcim20081106. [DOI] [PubMed] [Google Scholar]
- 17.Li Y, Chen K, Shi Z. Effect of xuefu zhuyu pill on blood stasis syndrome and risk factor of atherosclerosis. Zhongguo Zhong Xi Yi Jie He Za Zhi. 1998;18(2):71–73. [PubMed] [Google Scholar]
- 18.Shiyun HU, Shaoxiang X, Licheng Z. Effect of tianma gouteng decoction on myocardial fibrosis in renovascular hypertensive rats. Traditional Chinese drug research & clinical pharmacology. 2006;17(2):97–99. [Google Scholar]
- 19.Zhang TX, Wang YF, Ciriello J. The herbal medicine tian ma gou teng yen alters the development of high blood pressure in the spontaneously hypertensive rat. Am J Chin Med. 1989;17(3–4):211–219. doi: 10.1142/S0192415X89000309. [DOI] [PubMed] [Google Scholar]
- 20.Zhong-qiang JI, Wen-hui WU, Wen-yan JI. Mechanism study of the impacts on myocardial fibrosis treated with modified xuefu zhuyu decoction in the rats with hypertension. World journal of integrated of traditional and western medicine. 2011;06(5):385–388. [Google Scholar]
- 21.Gao D, Jiao YH, Wu YM. Experimental study of Xuefu Zhuyu Decoction induced participation of endothelial progenitor cells in the angiogenesis of the ischemic region. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2012;32(2):224–228. [PubMed] [Google Scholar]
- 22.Guohua Z. Effect of Wendan decoction on lipid metabolism in hyperlipidemic adult rats. Hebei journal of traditional Chinese medicine. 2007;7(5):28–29. [Google Scholar]
- 23.Wang L, Song Y, Li F, Liu Y, Ma J, Mao M, et al. Effects of Wen Dan Tang on insomnia-related anxiety and levels of the brain-gut peptide Ghrelin. Neural regeneration research. 2014;9(2):205–212. doi: 10.4103/1673-5374.125351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fritz M, Rinaldi G. Blood pressure measurement with the tail-cuff method in Wistar and spontaneously hypertensive rats: influence of adrenergic- and nitric oxide-mediated vasomotion. J Pharmacol Toxicol Methods. 2008;58(3):215–221. doi: 10.1016/j.vascn.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 25.Jia H, Chen XL, Chen C, Hu YY, Yun XJ. Baicalin prevents the up-regulation of connective tissue growth factor in fibrotic lungs of rats. Sheng Li Xue Bao. 2010;62(6):535–540. [PubMed] [Google Scholar]
- 26.Zhai Y, Gao X, Wu Q, Peng L, Lin J, Zuo Z. Fluvastatin decreases cardiac fibrosis possibly through regulation of TGF-beta(1)/Smad 7 expression in the spontaneously hypertensive rats. Eur J Pharmacol. 2008;587(1–3):196–203. doi: 10.1016/j.ejphar.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 27.Akashiba A, Ono H, Ono Y, Ishimitsu T, Matsuoka H. Valsartan improves L-NAME-exacerbated cardiac fibrosis with TGF-ss inhibition and apoptosis induction in spontaneously hypertensive rats. J Cardiol. 2008;52(3):239–246. doi: 10.1016/j.jjcc.2008.07.018. [DOI] [PubMed] [Google Scholar]
- 28.Haider AW, Larson MG, Benjamin EJ, Levy D. Increased left ventricular mass and hypertrophy are associated with increased risk for sudden death. J Am Coll Cardiol. 1998;32(5):1454–1459. doi: 10.1016/S0735-1097(98)00407-0. [DOI] [PubMed] [Google Scholar]
- 29.Assayag P, Carre F, Chevalier B, Delcayre C, Mansier P, Swynghedauw B. Compensated cardiac hypertrophy: arrhythmogenicity and the new myocardial phenotype. I. Fibrosis. Cardiovasc Res. 1997;34(3):439–444. doi: 10.1016/S0008-6363(97)00073-4. [DOI] [PubMed] [Google Scholar]
- 30.Fortuno MA, Ravassa S, Fortuno A, Zalba G, Diez J. Cardiomyocyte apoptotic cell death in arterial hypertension: mechanisms and potential management. Hypertension. 2001;38(6):1406–1412. doi: 10.1161/hy1201.099615. [DOI] [PubMed] [Google Scholar]
- 31.Diez J. Mechanisms of cardiac fibrosis in hypertension. J Clin Hypertens. 2007;9(7):546–550. doi: 10.1111/j.1524-6175.2007.06626.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Caglayan E, Stauber B, Collins AR, Lyon CJ, Yin F, Liu J, et al. Differential roles of cardiomyocyte and macrophage peroxisome proliferator-activated receptor gamma in cardiac fibrosis. Diabetes. 2008;57(9):2470–2479. doi: 10.2337/db07-0924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rossi MA, Peres LC. Effect of captopril on the prevention and regression of myocardial cell hypertrophy and interstitial fibrosis in pressure overload cardiac hypertrophy. Am Heart J. 1992;124(3):700–709. doi: 10.1016/0002-8703(92)90281-Y. [DOI] [PubMed] [Google Scholar]
- 34.Rodriguez-Vita J, Sanchez-Lopez E, Esteban V, Ruperez M, Egido J, Ruiz-Ortega M. Angiotensin II activates the Smad pathway in vascular smooth muscle cells by a transforming growth factor-beta-independent mechanism. Circulation. 2005;111(19):2509–2517. doi: 10.1161/01.CIR.0000165133.84978.E2. [DOI] [PubMed] [Google Scholar]
- 35.Simko F. Physiologic and pathologic myocardial hypertrophy--physiologic and pathologic regression of hypertrophy? Med Hypotheses. 2002;58(1):11–14. doi: 10.1054/mehy.2001.1399. [DOI] [PubMed] [Google Scholar]
- 36.Cohn JN, Ferrari R, Sharpe N. Cardiac remodeling--concepts and clinical implications: a consensus paper from an international forum on cardiac remodeling. Behalf of an International Forum on Cardiac Remodeling. J Am Coll Cardiol. 2000;35(3):569–582. doi: 10.1016/S0735-1097(99)00630-0. [DOI] [PubMed] [Google Scholar]
- 37.Massague J. The transforming growth factor-beta family. Annu Rev Cell Biol. 1990;6:597–641. doi: 10.1146/annurev.cb.06.110190.003121. [DOI] [PubMed] [Google Scholar]
- 38.Dobaczewski M, Chen W, Frangogiannis NG. Transforming growth factor (TGF)-beta signaling in cardiac remodeling. J Mol Cell Cardiol. 2011;51(4):600–606. doi: 10.1016/j.yjmcc.2010.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhishan D, Chengxian G, Ruirui J, Xingde W. Effects of XueFuZhuYu decoction on collagen synthesis and proliferation of cardiac fibroblasts. Journal of Chinese Medicinal Materials. 2002;07:481–483. [PubMed] [Google Scholar]
- 40.\Miya Y, Jingping O, Yanfang Z: Effects of Sodiumferulate on the Proliferation of Cardiac Fibroblasts. Medical Journal of Wuhan University 2006(02):148-151+278.
- 41.Hailing R, Shisen J, Dujiang X, Chunhui W, Tao H: Effect of Ligustrazine on myocardial fibrosis in rats with pressure overload. Mod Rehab. 2003;(12):1748-1749+1875.
- 42.Fa L, Yuan W, Xinzhong Y, Fanggui L, Jian H, Ruifen C. The antihypertensive effect of carthamin yellow and the impact on renin angiotensin in hypertensive rats. Acta Pharmaceutica Sinica. 1992;10:785–787. [Google Scholar]
- 43.Guangsheng Y, Mingui T, Wangchun R, Jiahe H, Zhabang Z, Yongzhen H, et al. Peach kernel research for the treatment of schistosomiasis cirrhosis. J Tradit Chin Med. 1986;06:24–25. [Google Scholar]
- 44.Chunfu W. Research on the pharmacological of peony and its chemical constituents. Traditional Chinese Medicine Bulletin. 1985;06:45–47. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The protocol of animal experimental. (DOCX 796 kb)
The approval of the Southern Medical University Animal Cave and Use Committee. (DOCX 1607 kb)
The constitution of the Southern Medical University Animal Cave and Use Committee. (DOCX 3226 kb)
The staff of the Southern Medical University Animal Cave and Use Committee. (DOCX 658 kb)
The HPLC results for the quality control of the herbs. (PDF 27 kb)


