Abstract
Purpose
The incidental detection of early-stage kidney tumors is increasing in the United States. Nephron-sparing approaches (NS) to managing these tumors are equivalent to radical nephrectomy (RN) in oncologic outcomes and have a decreased impact on renal function. Our objective was to evaluate trends in the use of NS over the past decade and the socioeconomic factors associated with its use.
Methods
The National Cancer Database was queried to identify patients with stage I kidney cancer between 2000 and 2008. Patients were classified by the type of surgery as NS (local destruction and local excision) or RN. Patients were further categorized by age, race, insurance status, and income. Log-binomial regression was used to estimate prevalence ratios (PR) for the proportion of NS to RN according to demographic and socioeconomic characteristics.
Results
From 2000 to 2008, there were 142,194 cases of kidney cancer reported to the NCDB. In these cases, 43,034 (30.3 %) patients had NS, and 86,431 (60.78 %) patients had RN. The prevalence of NS increased 10 % per year (PR = 1.10, p < 0.0001)—from 20.0 % in 2000 to 45.1 % in 2008. Older age, lower income, Black race, Hispanic ethnicity, and lack of health insurance were associated with a decreased prevalence of NS.
Conclusions
NS as a treatment for stage I kidney cancer has increased steadily since 2000. Age, racial, and socioeconomic differences may exist in the utilization of NS. Additional analyses, with patient level data, are required to address the independent significance of these variables in an effort to develop strategies to mitigate these potential disparities.
Keywords: Renal surgery, Nephrectomy, Nephron-sparing surgery, Kidney cancer
Introduction
In 2012, there will be 64,770 new cases of kidney cancer in the United States [1]. Since the mid-1990s, there has been a downward stage migration in kidney cancer diagnosis, as the incidental detection of early-stage tumors has increased with the widespread use of cross-sectional diagnostic imaging [2]. Throughout this period, the use of nephron-sparing procedures (NS), including partial nephrectomy (PN) and local tumor ablation, has gained popularity over radical nephrectomy (RN) for the treatment of stage I (<7 cm) tumors [3–8].
PN and RN provide similar oncologic outcomes [9, 10]. In 2011, the EORTC randomized trial of RN versus NS in patients with T1-2 renal tumors showed a slight overall survival advantage with RN; however, in patients with renal cell carcinoma only, there was no significant difference in oncologic outcomes between the procedures [11]. Likewise, a Surveillance, Epidemiology, and End Results (SEER) analysis demonstrated comparable survival in patients with T1b lesions who underwent RN or NS [12]. Regardless, PN results in superior postoperative renal and cardiovascular function and an overall decrease in the risk of all-cause mortality [13–17]. Other NS ablative techniques, including radiofrequency ablation and cryoablation, and have shown similar short-term results [18, 19]. In 2009, the American Urological Association issued new guidelines for the treatment of small T1 kidney tumors suggesting that surgeons should consider NS approaches in all patients [20].
Previous single-institutional studies, multi-institutional studies, and population-based studies have shown that NS procedures are increasingly utilized in treating small renal masses [4–6]. These studies showed increased rates of NS from 11 to 20 % in early 2000 to 25–98 % in 2007–2008. Despite this trend, the studies recognized an overall under-utilization of NS and possible disparities in its implementation. As the National Cancer Database (NCDB) includes approximately 75 % of individuals diagnosed with cancer in the United States from 1,400 hospitals, we aimed to use this resource to investigate the trends of NS and RN for the treatment of stage I kidney tumors from 2000 to 2008 and to generate hypotheses regarding socioeconomic and regional differences that are uniquely captured by this database.
Materials and methods
Data were obtained from the NCDB Public dataset (http://cromwell.facs.org/BMarks/BMPub/Ver10/bm_reports.cfm). The NCDB is a national, hospital-based cancer registry sponsored by the American College of Surgeons Commission on Cancer (CoC) and the American Cancer Society. Since 1996, all CoC-accredited programs have been required to report cancers diagnosed or treated at their facilities to the NCDB. The NCDB contains standardized data elements on patient demographics, tumor characteristics, surgery performed, and the first course of treatment. In addition, the NCDB contains information on patient insurance, location of residence, income level, and type of treatment facility. The NCDB Public dataset allows access to aggregate data categorized by multiple variables but not to individual patient level data [21]. Because no protected health information was present in the dataset, institutional review board approval was not required for this study.
Study participants were diagnosed with American Joint Committee on Cancer (AJCC) stage I kidney cancer and underwent partial nephrectomy, hemi-nephrectomy or local ablation (NS procedures) or RN. Age at diagnosis, race/ethnicity, sex, and insurance type were categorically analyzed. Data regarding stage, year of diagnosis, and surgery were extracted. Of note, the NCDB combines cases of kidney and renal pelvis cancer in the same category. As a result, 4.4 % of our selected sample (n = 6,300) had transitional cell carcinoma (TCC), and because only aggregate data can be extracted from the NCDB Public dataset, these patients could not be excluded from the analysis. Area-based indicators of patient socioeconomic status, specifically education and income, were derived from United States census data at the zip code level and divided into quartiles based on the observed distribution. The proportions of the population in the patient's zip code of residence without a high school degree are divided into the following groups: >29, 20–28.9, 14–19.9, and <14 %.
Four types of treatment facilities are described: teaching/research centers, community hospitals, comprehensive community cancer centers, and VA hospitals. Teaching/research facilities have residency programs and active clinical research. Community hospitals treat at least 300 cancer cases each year and have a full range of services for cancer care. Community cancer centers offer the same range of services as community hospitals, but have at least 750 annual cancer cases.
Log-binomial regression was used to estimate prevalence ratios (PR) and corresponding 95 % confidence intervals (CIs) relating the proportions of patients having NS and RN for stage I tumors among categories of various socioeconomic and demographic variables. All statistical analyses were performed using SAS Version 9.2 (SAS Institute Inc., Cary, NC, USA).
Results
In total, 292,668 patients diagnosed with kidney cancer between 2000 and 2008 were extracted from the NCDB. Of these, 142,194 had stage I tumors. Overall, 43,034 patients had NS (30.3 %) and 86,431 patients had RN (60.8 %), and 7,539 patients had no surgery of the primary tumor site (5.3 %) and 5,190 had other, unspecified, or unknown procedures (3.7 %). These patients were excluded from the analysis. Of the 43,034 total NS procedures, 3,778 (8.8 %) were local destruction and 39,256 (91.2 %) were local, partial or subtotal nephrectomy procedures.
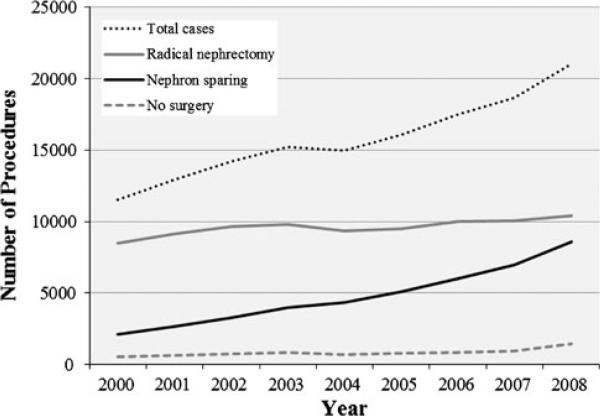
The prevalence of NS over RN increased 10 % per year between 2000 and 2008 (PR = 1.10, p < 0.0001). The total number of stage I kidney cancers reported also increased between 2000 and 2008 by about 1,045 patients per year (p < 0.0001). In 2000, there were 11,538 stage I tumors with 2,118 (18.4 %) NS, and in 2008, there were 21,033 stage I tumors with 8,571 (40.8 %) NS (Fig. 1).
Fig. 1.
Total stage I kidney cancers, radical nephrectomy procedures, nephron-sparing procedures and no surgical treatment cases between 2000 and 2008
Patient demographics and prevalence ratios are presented in Tables 1 and 2. Age was a major factor in the prevalence of NS. With every additional 10 years of age, the prevalence of NS decreased 5 % (PR = 0.95, p < 0.0001). In our cohort, the proportion of patients receiving NS was 41.0, 34.4, 32.6, and 27.0 % of patients <40, 40–60, 60–80, and >80, respectively. Men were 11 % more likely to receive an NS procedure than women (PR = 1.11, p < 0.0001). Blacks and Hispanics were 3 % (p = 0.048) and 10 % (p < 0.0001) less likely, respectively, to undergo NS, compared with whites. Asian/Pacific Islanders (p = 0.51) and Native Americans (p = 0.75) did not have a significantly different rate of NS than whites.
Table 1.
Characteristics of patients receiving nephron sparing and radical nephrectomy
| Nephron sparing (N) | Percent | Radical nephrectomy (N) | Percent | |
|---|---|---|---|---|
| Age | ||||
| Under 20 | 46 | 18.9 | 198 | 81.1 |
| 20–29 | 482 | 48.6 | 510 | 51.4 |
| 30–39 | 2,207 | 40.6 | 3,231 | 59.4 |
| 40–49 | 5,946 | 34.9 | 11,075 | 65.1 |
| 50–59 | 10,573 | 34.1 | 20,465 | 65.9 |
| 60–69 | 11,931 | 34.1 | 23,088 | 65.9 |
| 70–79 | 9,147 | 30.8 | 20,546 | 69.2 |
| 80–89 | 2,613 | 27.0 | 7,057 | 73.0 |
| 90 and over | 89 | 25.4 | 261 | 74.6 |
| Gender | ||||
| Male | 27,163 | 34.6 | 51,315 | 65.4 |
| Female | 15,871 | 31.1 | 35,116 | 68.9 |
| Race | ||||
| White | 34,628 | 33.4 | 69,189 | 66.6 |
| Black | 4,620 | 32.5 | 9,588 | 67.5 |
| Hispanic | 2,039 | 30.1 | 4,743 | 69.9 |
| API | 629 | 32.6 | 1,298 | 67.4 |
| Native American | 155 | 34.1 | 300 | 65.9 |
| Other/unknown | 963 | 42.3 | 1,313 | 57.7 |
| Insurance status | ||||
| Private insurance | 5,873 | 33.1 | 11,874 | 66.9 |
| Managed care | 15,461 | 36.4 | 26,992 | 63.6 |
| Medicaid | 1,587 | 32.0 | 3,370 | 68.0 |
| Medicare | 16,460 | 30.2 | 38,036 | 69.8 |
| VA | 1,062 | 39.9 | 1,601 | 60.1 |
| Military | 420 | 39.3 | 648 | 60.7 |
| Indian/public health service | 37 | 24.5 | 114 | 75.5 |
| Not insured | 790 | 28.7 | 1,962 | 71.3 |
| Unknown | 1,344 | 42.3 | 1,834 | 57.7 |
| Median income | ||||
| Less than $28,000 | 3,599 | 30.3 | 8,290 | 69.7 |
| $28,000–$32,999 | 4,818 | 30.1 | 11,178 | 69.9 |
| $33,000–$38,999 | 7,304 | 31.9 | 15,590 | 68.1 |
| $39,000–$48,999 | 9,821 | 33.2 | 19,719 | 66.8 |
| $49,000 or greater | 14,835 | 35.8 | 26,636 | 64.2 |
| Unknown | 2,657 | 34.6 | 5,018 | 65.4 |
| Percent without high school education | ||||
| 31 % or greater | 5,033 | 30.1 | 11,691 | 69.9 |
| 23–30.9 % | 6,746 | 31.7 | 14,514 | 68.3 |
| 18–22.9 % | 6,840 | 32.9 | 13,968 | 67.1 |
| 12–17.9 % | 10,323 | 33.5 | 20,489 | 66.5 |
| Less than 12 % | 11,432 | 35.5 | 20,742 | 64.5 |
| Unknown | 2,660 | 34.6 | 5,027 | 65.4 |
| Hospital type | ||||
| Teaching/research | 21,229 | 43.5 | 27,550 | 56.5 |
| Community | 3,334 | 22.7 | 11,361 | 77.3 |
| Comprehensive community | 16,394 | 27.5 | 43,268 | 72.5 |
| VA | 1,280 | 40.7 | 1,865 | 59.3 |
| Other | 797 | 25.0 | 2,387 | 75.0 |
API Asian/Pacific Islander, VA veterans affairs
Table 2.
Impact of patient demographics and treatment facility on the use of NS procedures
| Variable | Unit | Prevalence ratio | Confidence limits | p value | |
|---|---|---|---|---|---|
| Age | 10 years | 0.95 | 0.94 | 0.95 | <0.0001* |
| Gender | Women | Ref | – | – | – |
| Men | 1.11 | 1.09 | 1.13 | <0.0001* | |
| Race | White | Ref | – | – | – |
| Black | 0.98 | 0.95 | 1.00 | 0.05* | |
| Hispanic | 0.90 | 0.87 | 0.94 | <0.0001* | |
| API | 0.98 | 0.92 | 1.04 | 0.51 | |
| Native American | 1.02 | 0.90 | 1.16 | 0.75 | |
| Other | 1.27 | 1.21 | 1.33 | <0.0001* | |
| Insurance | Private | Ref | – | – | – |
| Managed care | 1.10 | 1.07 | 1.13 | <0.0001* | |
| Medicaid | 0.97 | 0.92 | 1.01 | 0.16 | |
| Medicare | 0.91 | 0.89 | 0.94 | <0.0001* | |
| VA | 1.21 | 1.15 | 1.27 | <0.0001* | |
| Military | 1.19 | 1.10 | 1.28 | <0.0001* | |
| Indian/public health service | 0.74 | 0.56 | 0.98 | 0.04 | |
| None | 0.87 | 0.82 | 0.92 | <0.0001* | |
| Unknown | 1.28 | 1.22 | 1.34 | <0.0001* | |
| Median income | $10,000 increase | 1.07 | 1.06 | 1.08 | <0.0001* |
| Percent without high school education | 5 % increase (i.e., less education) | 0.96 | 0.96 | 0.97 | <0.0001* |
| Hospital type | Teaching/research | Ref | – | – | – |
| Community | 0.52 | 0.51 | 0.54 | <0.0001* | |
| Comprehensive community | 0.63 | 0.62 | 0.64 | <0.0001* | |
| VA | 0.94 | 0.90 | 0.98 | 0.003* | |
| Other | 0.58 | 0.54 | 0.61 | <0.0001* |
VA Veterans Affairs, Ref referent
There were also significant differences in NS utilization by insurance status. Patients insured with Medicare were 9 % less likely (p < 0.0001) and those uninsured were 13 % less likely (p < 0.0001) to have NS than patients with private insurance. Patients with Medicaid did not have a significant difference in the prevalence of NS than those with private insurance (PR = 0.97, p = 0.15). Other patients insured with managed care (PR = 1.10, p < 0.0001), military insurance (PR = 1.19, p < 0.0001), and VA insurance (PR = 1.21, p < 0.0001) were significantly more likely to have NS than those with private insurance.
Low income and low education level were associated with decreased use of NS. For every additional $10,000 of median annual income, the prevalence of NS increased 7 % (p < 0.0001). With every 5 % increase in the proportion of people without a high school education, the prevalence of NS decreased 4 % (p < 0.0001, Table 2).
Finally, there were significant differences in the prevalence of NS by hospital type. Patients treated at community hospitals were 48 % less likely to have NS than those treated at teaching hospitals (p < 0.0001). Patients treated at community comprehensive community cancer programs were 37 % less likely to have NS (p < 0.0001) than patients treated at teaching hospitals. Patients treated at VA hospitals (PR = 0.94, p = 0.003) and “other” hospitals (PR = 0.58, p < 0.0001) were also less likely to have NS than those treated at teaching hospitals.
Discussion
Given the similar anticancer outcomes and decreased morbidity compared with RN, NS procedures are now becoming widely adopted for the surgical management of stage I kidney tumors. In this study, we sought to determine the practice patterns of NS over the past decade and to identify socioeconomic differences in the use of NS over RN. Our analysis showed an increase in NS procedures from 18.4 % in 2000 to 40.8 % in 2008. Importantly, our study included 142,194 patients and is among the largest cohorts to date to address this trend. Despite the increased uptake of NS, nearly 60 % of patients are still receiving a treatment that is associated with increased treatment-related morbidity, and perhaps, treatment-related mortality.
The results of our study provide further evidence for the increasing adoption of NS. Previous studies have shown that NS procedures are increasingly utilized in treating small renal masses [4–6, 22] An analysis from SEER data consisting of approximately 20,000 patients showed an increase in the use of partial nephrectomy for stage I and II tumors from 5.3 to 23% from 1989 to 2004 [23]. Two analyses of the Nationwide Inpatient Sample (NIS), demonstrated consistent increases in the use of PN for kidney tumors from 16.8 % in 2003 to 25.1 % in 2008 (N = 188,702) and from 15.3 % in 2002 to 24.7 % in 2008 (N = 46,396), respectively [4, 22]. Since local tumor destruction is becoming increasingly popular for the treatment of small renal masses in addition to PN, we chose to group these procedures together to capture the most accurate representation of their national utilization.
We identified several potential demographic and socioeconomic differences in the use of NS over RN during this time period. The prevalence of NS decreased with advancing patient age. Kates et al. [24] previously showed persistent underuse NS in the elderly, which may be related to an assumption that elderly patients do not benefit from kidney preservation or the desire to avoid more complicated surgery in patients with increased comorbidities. However, studies have shown that after RN, renal function decline occurs within the first 2 years and that PN and RN comparable postoperative complication rates in older patients [25, 26]. Thus, elderly patients with life expectancy greater than 2 years may benefit from NS as much as younger patients. Gender also was associated with differences in the use of NS. Men were more likely to receive NS procedures than women, which is consistent with previous studies [23].
We found that Blacks and Hispanics were significantly less likely to undergo NS compared with Whites. The underlying biology of disease and decreased accessibility to optimal care may also be critical factors, as has been demonstrated with other therapeutic modalities in studies of various solid tumors [27, 28]. Prior studies have demonstrated that blacks have a higher incidence of renal carcinoma and lower survival rates [29]. We also found differential utilization of NS by insurance status, income, and education level. Patients with Medicare or no insurance were less likely to undergo NS than those with private insurance. Decreased levels of insurance, education, and income likely contribute to poor accessibility to and acceptability of novel cancer treatments and have previously been correlated with worse outcomes in patients with cancer [30].
Finally, we found significant differences in the prevalence of NS utilization by hospital type. Patel et al. [22] also showed that small, rural non-academic hospitals lagged behind large, urban academic hospitals in the use of PN over RN and that hospital volume is one of the strongest predictors of use of RN (OR 2.08 for highest volume vs. lowest volume hospitals, p < 0.001). These observed trends could be due to a variety of factors, including volume of fellowship trained surgeons at academic programs, access to minimally invasive technologies in certain regions, and patient demand for NS in urban centers.
A strength of our study is the use of a very large dataset representing a diverse population of patients and the focus on a contemporary time period when changes in practice patterns are rapidly altering the treatment of small renal masses. The NCDB is an excellent resource for creating quality of care reports because it includes data from a large variety of regions and hospital types considered representative of cancer care in the US population. However, our study has several limitations. Foremost, we utilized the Public NCDB dataset and did not have access to individual patient data or clinical information regarding tumor characteristics and comorbidities. Therefore, we could not perform multivariable analysis to determine which seemingly related variables associated with decreased NS retain independent significance and we could not independently assess patients that were candidates for NS procedures. In combining PN and ablative procedures for our analysis, we may have potentially overlooked trends within these individual NS procedures and trends in specific surgical approaches. Therefore, our findings can only be considered hypothesis-generating and further evaluation utilizing individual patient data is warranted. Our dataset includes a small subset of patients with TCC (4.4 %). The proportion of the patients with TCC was constant during the years 2001–2008 and therefore should not influence the overall surgical trends. Furthermore, patients with upper-tract TCC likely underwent total nephroureterectomy, thus the trend in NS may actually be underestimated. Of note, 5.3 % of the patients in this cohort had no surgery of the primary tumor site, suggesting that they were undergoing active surveillance, declined treatment, or were unable to tolerate surgery. The characteristics of this latter group of patients and their clinical outcomes merit further study. Although the NCDB includes data for >75 % of new cancer cases in the United States annually, CoC-approved hospitals often provide more cancer-related services (including cancer screening, chemotherapy, and radiation) than non-CoC-accredited hospitals so these findings may not be generalizable to non-CoC-accredited hospitals.
Conclusions
While there is an overall increasing trend in the proportion of stage I kidney tumors treated with NS instead of RN, NS remains underutilized. Racial/ethnic, socioeconomic and regional disparities may exist in the use of NS. Our analysis is limited by aggregate-level data, and thus our findings are hypothesis-generating. Further research is required to evaluate the barriers to accessibility and acceptability of NS.
Acknowledgments
Source of Funding: Doris Duke Charitable Foundation.
Footnotes
Conflict of interest The authors declare that they have no conflict of interest.
Contributor Information
Alexander C. Small, Division of Hematology and Medical Oncology, The Tisch Cancer Institute, Mount Sinai School of Medicine, 1 Gustave L Levy Place, New York, NY 10029, USA
Che-Kai Tsao, Division of Hematology and Medical Oncology, The Tisch Cancer Institute, Mount Sinai School of Medicine, 1 Gustave L Levy Place, New York, NY 10029, USA.
Erin L. Moshier, Division of Hematology and Medical Oncology, The Tisch Cancer Institute, Mount Sinai School of Medicine, 1 Gustave L Levy Place, New York, NY 10029, USA
Benjamin A. Gartrell, Division of Hematology and Medical Oncology, The Tisch Cancer Institute, Mount Sinai School of Medicine, 1 Gustave L Levy Place, New York, NY 10029, USA
Juan P. Wisnivesky, Division of General Internal Medicine, Mount Sinai School of Medicine, New York, NY, USA Division of Pulmonary and Critical Care Medicine, Mount Sinai School of Medicine, New York, NY, USA.
James Godbold, Division of Hematology and Medical Oncology, The Tisch Cancer Institute, Mount Sinai School of Medicine, 1 Gustave L Levy Place, New York, NY 10029, USA.
Guru Sonpavde, Texas Oncology, Houston, TX, USA; Department of Medicine, Section of Medical Oncology, Michael E. DeBakey Veterans Affairs Medical Center, Baylor College of Medicine, Houston, TX, USA.
Michael A. Palese, Department of Urology, Mount Sinai School of Medicine, New York, NY, USA
Simon J. Hall, Department of Urology, Mount Sinai School of Medicine, New York, NY, USA
William K. Oh, Division of Hematology and Medical Oncology, The Tisch Cancer Institute, Mount Sinai School of Medicine, 1 Gustave L Levy Place, New York, NY 10029, USA
Matthew D. Galsky, Division of Hematology and Medical Oncology, The Tisch Cancer Institute, Mount Sinai School of Medicine, 1 Gustave L Levy Place, New York, NY 10029, USA
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Kane CJ, Mallin K, Ritchey J, Cooperberg MR, Carroll PR. Renal cell cancer stage migration: analysis of the National Cancer Data Base. Cancer. 2008;113:78–83. doi: 10.1002/cncr.23518. [DOI] [PubMed] [Google Scholar]
- 3.Gill IS, Aron M, Gervais DA, Jewett MAS. Clinical practice. Small renal mass. N Engl J Med. 2010;362:624–634. doi: 10.1056/NEJMcp0910041. [DOI] [PubMed] [Google Scholar]
- 4.Kim SP, Shah ND, Weight CJ, Thompson RH, Moriarty JP, Shippee ND, Costello BA, Boorjian SA, Leibovich BC. Contemporary trends in nephrectomy for renal cell carcinoma in the United States: results from a population based cohort. J Urol. 2011;186:1779–1785. doi: 10.1016/j.juro.2011.07.041. [DOI] [PubMed] [Google Scholar]
- 5.Lane BR, Chen H, Morrow M, Anema JG, Kahnoski RJ. Increasing use of kidney sparing approaches for localized renal tumors in a community based health system: impact on renal functional outcomes. J Urol. 2011;186:1229–1235. doi: 10.1016/j.juro.2011.05.081. [DOI] [PubMed] [Google Scholar]
- 6.Thompson RH, Kaag M, Vickers A, Kundu S, Bernstein M, Lowrance W, Galvin D, Dalbagni G, Touijer K, Russo P. Contemporary use of partial nephrectomy at a tertiary care center in the United States. J Urol. 2009;181:993–997. doi: 10.1016/j.juro.2008.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miller DC, Hollingsworth JM, Hafez KS, Daignault S, Hollenbeck BK. Partial nephrectomy for small renal masses: an emerging quality of care concern? J Urol. 2006;175:853–857. doi: 10.1016/S0022-5347(05)00422-2. (discussion 858) [DOI] [PubMed] [Google Scholar]
- 8.Cooperberg MR, Mallin K, Kane CJ, Carroll PR. Treatment trends for stage I renal cell carcinoma. J Urol. 2011;186:394–399. doi: 10.1016/j.juro.2011.03.130. [DOI] [PubMed] [Google Scholar]
- 9.Uzzo RG, Novick AC. Nephron sparing surgery for renal tumors: indications, techniques and outcomes. J Urol. 2001;166:6–18. [PubMed] [Google Scholar]
- 10.Leibovich BC, Blute ML, Cheville JC, Lohse CM, Weaver AL, Zincke H. Nephron sparing surgery for appropriately selected renal cell carcinoma between 4 and 7 cm results in outcome similar to radical nephrectomy. J Urol. 2004;171:1066–1070. doi: 10.1097/01.ju.0000113274.40885.db. [DOI] [PubMed] [Google Scholar]
- 11.Van Poppel H, Da Pozzo L, Albrecht W, Matveev V, Bono A, Borkowski A, Colombel M, Klotz L, Skinner E, Keane T, et al. A prospective, randomised EORTC intergroup phase 3 study comparing the oncologic outcome of elective nephron-sparing surgery and radical nephrectomy for low-stage renal cell carcinoma. Eur Urol. 2011;59:543–552. doi: 10.1016/j.eururo.2010.12.013. [DOI] [PubMed] [Google Scholar]
- 12.Badalato GM, Kates M, Wisnivesky JP, RoyChoudhury A, McKiernan JM. Survival after partial and radical nephrectomy for the treatment of stage T1bN0M0 renal cell carcinoma (RCC) in the USA: a propensity scoring approach. BJU Int. 2011 doi: 10.1111/j.1464-410X.2011.10597.x. doi:10.1111/j.1464-410X.2011.10597.x. [DOI] [PubMed] [Google Scholar]
- 13.Miller DC, Schonlau M, Litwin MS, Lai J, Saigal CS, Urologic Diseases in America Project Renal and cardiovascular morbidity after partial or radical nephrectomy. Cancer. 2008;112:511–520. doi: 10.1002/cncr.23218. [DOI] [PubMed] [Google Scholar]
- 14.Huang WC, Elkin EB, Levey AS, Jang TL, Russo P. Partial nephrectomy versus radical nephrectomy in patients with small renal tumors–is there a difference in mortality and cardiovascular outcomes? J Urol. 2009;181:55–61. doi: 10.1016/j.juro.2008.09.017. (discussion 61–62) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weight CJ, Larson BT, Gao T, Campbell SC, Lane BR, Kaouk JH, Gill IS, Klein EA, Fergany AF. Elective partial nephrectomy in patients with clinical T1b renal tumors is associated with improved overall survival. Urology. 2010;76:631–637. doi: 10.1016/j.urology.2009.11.087. [DOI] [PubMed] [Google Scholar]
- 16.McKiernan J, Simmons R, Katz J, Russo P. Natural history of chronic renal insufficiency after partial and radical nephrectomy. Urology. 2002;59:816–820. doi: 10.1016/s0090-4295(02)01501-7. [DOI] [PubMed] [Google Scholar]
- 17.Thompson RH, Boorjian SA, Lohse CM, Leibovich BC, Kwon ED, Cheville JC, Blute ML. Radical nephrectomy for pT1a renal masses may be associated with decreased overall survival compared with partial nephrectomy. J Urol. 2008;179:468–471. doi: 10.1016/j.juro.2007.09.077. (discussion 472–473) [DOI] [PubMed] [Google Scholar]
- 18.Gill IS, Remer EM, Hasan WA, Strzempkowski B, Spaliviero M, Steinberg AP, Kaouk JH, Desai MM, Novick AC. Renal cryoablation: outcome at 3 years. J Urol. 2005;173:1903–1907. doi: 10.1097/01.ju.0000158154.28845.c9. [DOI] [PubMed] [Google Scholar]
- 19.Pavlovich CP, Walther MM, Choyke PL, Pautler SE, Chang R, Linehan WM, Wood BJ. Percutaneous radio frequency ablation of small renal tumors: initial results. J Urol. 2002;167:10–15. [PMC free article] [PubMed] [Google Scholar]
- 20.Campbell SC, Novick AC, Belldegrun A, Blute ML, Chow GK, Derweesh IH, Faraday MM, Kaouk JH, Leveillee RJ, Matin SF, et al. Guideline for management of the clinical T1 renal mass. J Urol. 2009;182:1271–1279. doi: 10.1016/j.juro.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 21.Winchester DP, Stewart AK, Phillips JL, Ward EE. The national cancer data base: past, present, and future. Ann Surg Oncol. 2010;17:4–7. doi: 10.1245/s10434-009-0771-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Patel SG, Penson DF, Pabla B, Clark PE, Cookson MS, Chang SS, Herrell SD, Smith JA, Barocas DA. National trends in the use of partial nephrectomy: a rising tide that has not lifted all boats. J Urol. 2012;187:816–821. doi: 10.1016/j.juro.2011.10.173. [DOI] [PubMed] [Google Scholar]
- 23.Baillargeon-Gagné S, Jeldres C, Lughezzani G, Sun M, Isbarn H, Capitanio U, Shariat SF, Crépel M, Alasker A, Widmer H, et al. A comparative population-based analysis of the rate of partial vs radical nephrectomy for clinically localized renal cell carcinoma. BJU Int. 2010;105:359–364. doi: 10.1111/j.1464-410X.2009.08745.x. [DOI] [PubMed] [Google Scholar]
- 24.Kates M, Badalato G, Pitman M, McKiernan J. Persistent overuse of radical nephrectomy in the elderly. Urology. 2011;78:555–559. doi: 10.1016/j.urology.2011.02.066. [DOI] [PubMed] [Google Scholar]
- 25.Huang WC, Levey AS, Serio AM, Snyder M, Vickers AJ, Raj GV, Scardino PT, Russo P. Chronic kidney disease after nephrectomy in patients with renal cortical tumours: a retrospective cohort study. Lancet Oncol. 2006;7:735–740. doi: 10.1016/S1470-2045(06)70803-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Corman JM, Penson DF, Hur K, Khuri SF, Daley J, Henderson W, Krieger JN. Comparison of complications after radical and partial nephrectomy: results from the National Veterans Administration Surgical Quality Improvement Program. BJU Int. 2000;86:782–789. doi: 10.1046/j.1464-410x.2000.00919.x. [DOI] [PubMed] [Google Scholar]
- 27.Newman LA, Carolin K, Simon M, Kosir M, Hyrniuk W, Demers R, Grossbart Schwartz A, Visscher D, Peters W, Bouwman D. Impact of breast carcinoma on African-American women: the Detroit experience. Cancer. 2001;91:1834–1843. doi: 10.1002/1097-0142(20010501)91:9<1834::aid-cncr1204>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 28.Hoffman RM, Gilliland FD, Eley JW, Harlan LC, Stephenson RA, Stanford JL, Albertson PC, Hamilton AS, Hunt WC, Potosky AL. Racial and ethnic differences in advanced-stage prostate cancer: the Prostate Cancer Outcomes Study. J Natl Cancer Inst. 2001;93:388–395. doi: 10.1093/jnci/93.5.388. [DOI] [PubMed] [Google Scholar]
- 29.Stafford HS, Saltzstein SL, Shimasaki S, Sanders C, Downs TM, Sadler GR. Racial/ethnic and gender disparities in renal cell carcinoma incidence and survival. J Urol. 2008;179:1704–1708. doi: 10.1016/j.juro.2008.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Albano JD, Ward E, Jemal A, Anderson R, Cokkinides VE, Murray T, Henley J, Liff J, Thun MJ. Cancer mortality in the United States by education level and race. J Natl Cancer Inst. 2007;99:1384–1394. doi: 10.1093/jnci/djm127. [DOI] [PubMed] [Google Scholar]