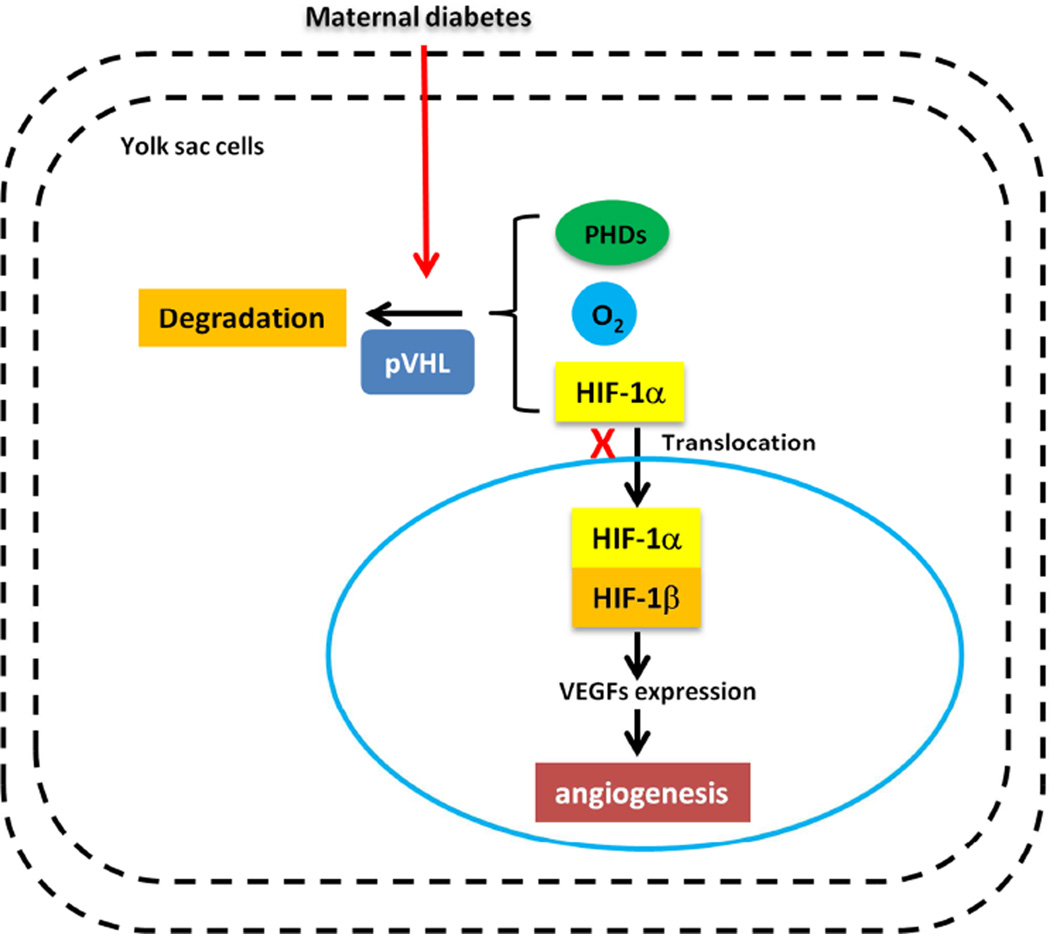
Figure 1. Maternal diabetes induces yolk sac vasculopathy via reduction of HIF-1α.
Under normoxic conditions, specific prolylhydroxylases (PHDs) induce oxygen-dependent hydroxylation of HIF-1α. HIF-1α then binds to the von Hippel-Lindau tumor suppressor protein (pVHL), which acts as an E3 ubiquitin ligase and targets HIF-1α for proteasomal degradation. Under hypoxic conditions, HIF-1α translocates to the nucleus, engages HIF-1β, and forms the HIF-1 complex that initiates transcription of downstream genes, including VEGFs. Maternal diabetes reduces HIF-1α levels by enhancing its degradation. The lack of HIF-1α leads to the development of extensive vascular defects, which is similar to diabetic yolk sac vasculopathy.