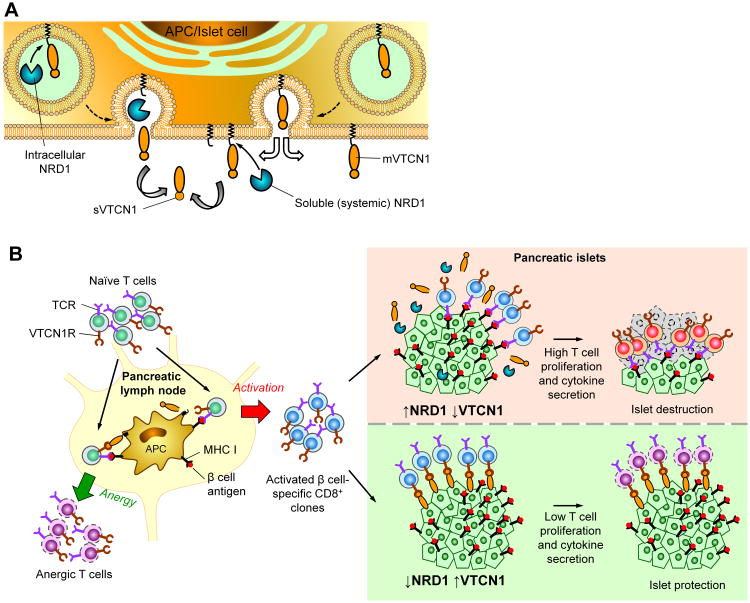
Figure 6. Schematic view of the impairment of VTCN1-dependent negative co-stimulation in context of T1D development.
(A) Cell-intrinsic and -extrinsic modes of VTCN1/NRD1 interactions. Cells with low internal levels of NRD1 display uninterrupted presentation of full length VTCN1 tethered to the cell membrane (mVTCN1), while high intracellular NRD1 production results in VTCN1 proteolysis and generation of soluble VTCN1 (sVTCN1) fragments. NRD1, secreted from high-producing surrounding cells, compliment VTCN1 proteolysis acting extracellularly. (B) Function of NRD1/VTCN1 axis in the development of T1D. Left, outcome of antigen-specific activation of islet-eactive T cells by APCs in pancreatic lymph nodes depends on NRD1/VTCN1 interplay. Low levels of intracellular and systemic NRD1 allow the continuous presentation of functional membrane-tethered VTCN1 by APCs, which provides negative co-stimulatory signal anergizing autoreactive T cells. When high NRD1 levels are present VTCN1 is being cleaved, negative co-stimulation is abrogated, and hyper-activation of autoreactive T cells is induced. Right, activated β cell-specific T cells migrate toward pancreatic islets and accumulate in islet infiltrates, where two potential scenarios, depending on NRD1 levels, occur: 1) Low NRD1 content and consequent high mVTCN1 levels on islet cells are protective, as VTCN1 signalling reduces proliferation and cytokine production of accumulating T cells; 2) Absence of mVTCN1 on the islets cells due to an increased NRD1-mediated shedding results in hyper proliferation of autoreactive T cells and consequent β cell destruction. VTCN1R – putative VTCN1 receptor.