Abstract
Neurological disorders of volition may be characterized by deficits in willing and/or agency. When we move our bodies through space, it is the sense that we intended to move (willing) and that our actions were a consequence of this intention (self-agency) that gives us the sense of voluntariness and a general feeling of being “in control.” While it is possible to have movements that share executive machinery ordinarily used for voluntary movement but lack a sense of voluntariness, such as psychogenic movement disorders, it is also possible to claim volition for presumed involuntary movements (early chorea) or even when no movement is produced (anosognosia). The study of such patients should enlighten traditional models of how the percepts of volition are generated in the brain with regards to movement. We discuss volition and its components as multi-leveled processes with feedforward and feedback information flow, and dependence on prior expectations as well as external and internal cues.
Introduction
Within the realm of clinical neurology, disorders are usually characterized by the location of the lesion, too much or too little movement, or the pathologic mechanism, and rarely by the degree of self-agency or will experienced by the patient. Most neurological patients are aware of volition only in that their life is fundamentally changed by the experience of not being able to will their bodies to do what they want or that their body is making movements that they do not want. The patient with stroke tries to will his paretic limb into moving again; the patient with epilepsy is suddenly overcome by seizure, beyond her control. In these examples, the forces of brain pathology and the patient’s will are clearly defined and directly at odds with each other. For many neurological patients, however, this differentiation is much less clear. This review will explore the neurological conditions at the borderland of voluntary and involuntary, with a view towards the insights into the nature of volition that might be gained from such patients.
The study of movement provides a conceptual framework with which to evaluate volition, since at least the results of one’s intentions when performing an action are clearly visible most of the time. While we may have many such intentions and failures on a cognitive basis (an example from prospective memory would be the failure of remembering to remember), the process of planning and executing a movement is amenable to clinical observation as well as electrophysiological or neuroimaging studies. The study of action is also at once immediate in its universality and relatability, since we all walk, and yet deceptively isolated from cognition, since we don’t have to think about the stepping motion in order to walk. For these reasons, movement becomes an ineluctable modality in the study of volition, and such philosophical investigations intersect with clinical neurology most often in the subspecialty of movement disorders. We will focus first on the movement disorders that deal with questions of volition, but also address certain non-movement neurological disorders as well.
An important conceptual framework in the study of neurological disorders of volition is the forward model. This model proposes that generation of the motor program in the brain also involves generation of an efference copy (or corollary discharge), which is then compared to proprioceptive feedback; if mismatch is detected between the feedback and the efference copy of the intended movement, the movement can be corrected even on-line. Additionally, the comparison of feedforward and feedback signals will lead to the perception that the movement was made according to plan, that is, it is voluntary, or that the movement was externally generated or involuntary (Blakemore et al., 2002, Frith et al., 2000). It is known that in making judgments or estimations, subjects will weigh input from different sensory modalities differently, with greatest weight given to the most reliable modality, usually vision. If visual feedback is disturbed, subjects will adapt by assigning greater weight to other sensory inputs (Ernst and Banks, 2002). In order to incorporate internal predictions (feedforward) with such external cues (feedback), estimates will be constantly updated as more information becomes available. Thus the reliability of external cues is weighted in estimation judgments, and these weights are further modified by prior information (the Bayesian “prior”), the top-down influence that would incorporate internal prediction models (see Figure 1) (Edwards et al., 2012, Moore and Fletcher, 2012). The magnitude of such top-down influences may be such, in certain pathological models, that random discharges or noise in the system might even be interpreted as meaningful stimuli (Edwards et al., 2012).
Figure 1.
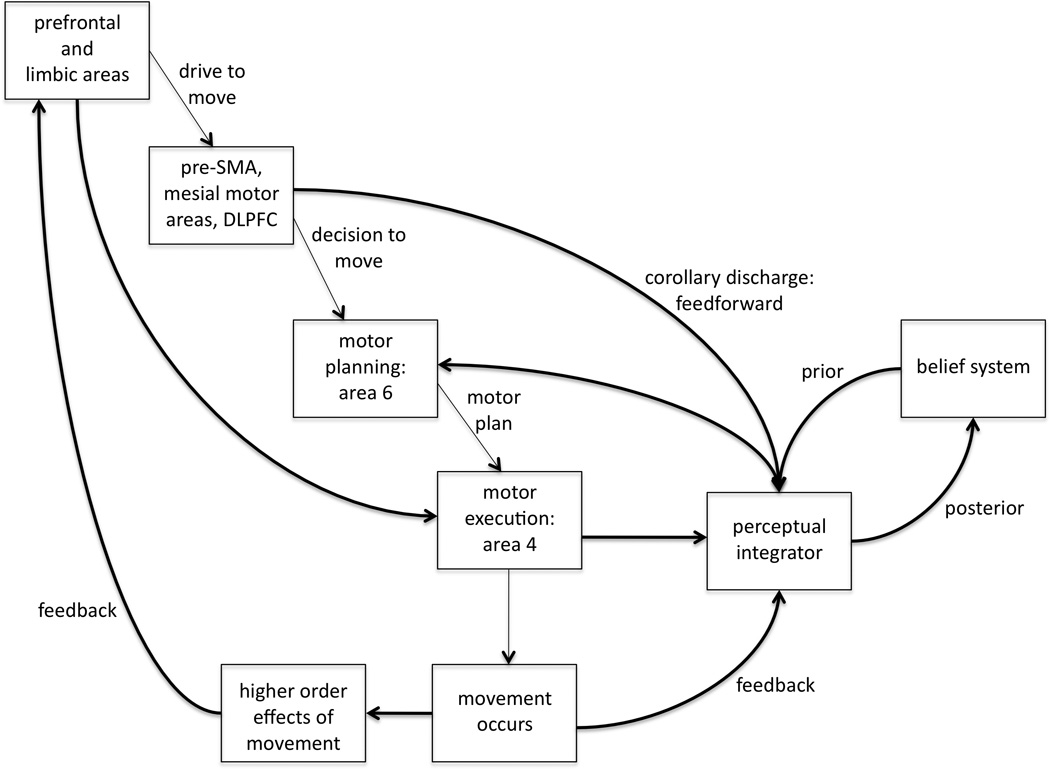
The forward model framework for volition. The sense of will reflects feedforward input from motor planning as well as prior beliefs. If the execution of the movement results in perceptual feedback that matches the program and the drive to move (or motor intention), the sense of agency is generated. See text for full explanation.
The accompanying diagram depicts this theoretical framework. There is a drive to move, as a result of limbic, homeostatic, or reward-related input (e.g., reaching for food when hungry). The motor program results in movement execution, but a copy of the program is also sent to a complex of brain regions that receive perceptual input (proprioceptive, visual, etc) for comparison. Feedforward input also comes from corollary discharge from other areas involved in motor planning, and the perceptual integrator is also influenced by the Bayesian prior of the subject’s belief system regarding volition. The sense of will likely reflects feedforward input from motor planning as well as prior beliefs. If the execution of the movement results in perceptual feedback that matches the program and the drive to move (or motor intention), the sense of agency is generated. Agency and will are thus subject to influence from prior beliefs and expectations, and as the sense of volition is updated with constant incoming perceptual information, a new Bayesian “posterior” is formed. The different neurological disorders described in this article are explained on the basis of this diagram.
Syndromes of Decreased Volition
Tics and Tourette Syndrome
Tics are sudden, quick, intermittent and repetitive movements or vocalizations. When multiple motor and vocal tics are present in a patient, beginning in childhood, criteria are met for the diagnosis of Tourette Syndrome. Tics are within a range of abnormal movements that are unified in their accompanied sensory abnormalities and disordered sense of volition. Patients with tics often have difficulty answering the question of “did you do that, or is that just happening to you.” Frequently the response is that a premonitory sensation or urge to move “just happens,” and will continue to build in intensity until the patient complies, performing a voluntary movement but feeling “no choice” but to do so, similar to an itch that must be scratched. While patients may differ in the degree of premonitory sensation reported as well as to other features such as suppressibility, these features help clinicians diagnose abnormal movements as tics. Much attention has focused in recent years on the heterogeneous phenomenology apparent in Tourette syndrome (TS); tics may range from simple motor acts such as blinking or shrugging to complex motor tasks that may resemble compulsions, such as intricate coordinated gestures or repetitive sequences of movements. Tics with varying degrees of complexity may co-exist in any given patient, and patients may report varying levels of “voluntariness” for different tics. There also appears to be a differing degree of awareness for tics on the part of the patient, with more complex motor tics occupying more of the patient’s awareness while simple brief tics can escape notice. The tic itself may be so quick as to appear involuntary to the clinical observer, but this is a function of practice in that these movements are performed so many times as to become automatic in their execution.
Electrophysiological studies have been performed in patients with tics in an attempt to determine if tics are preceded by the same electrical signature as is seen in voluntary movement. Studying six TS patients with simple motor tics who were also able to voluntarily imitate their tics, Obeso et al. found that the premovement EEG potential or motor-related cortical potential (MRCP) was clearly demonstrated prior to the imitations but not the tics (Obeso et al., 1981). Karp et al. analyzed EEG data from 5 patients during simple motor tics as well as imitated tics, two of whom classified their tics as involuntary and the other three as both involuntary and voluntary (Karp et al., 1996). Three patients showed MRCPs prior to the imitated tics only, and two of these patients demonstrated motor related cortical potentials (MRCPs) prior to their tics as well as the imitations. While early Bereitschaftspotentials (BPs) were not seen, the premotor potential in these patients resembled the late BP or BP2 potential in that these were rapidly rising negative potentials starting approximately 100–200 ms prior to the onset of movement (see Figure 2). This shortened version of the MRCP, lacking the early BP component, is similar to what has been shown in voluntary movements made in response to a triggering stimulus (Papa et al., 1991), and likely reflects mainly activity related to movement generation (see Figure 2). One of the two patients with MRCPs prior to their tics characterized the tics as entirely involuntary, while the other patient stated that tics were voluntarily performed in response to a clear internal urge (Karp et al., 1996). Hence, there was no relationship between the presence or absence of the potential and the sense of volition.
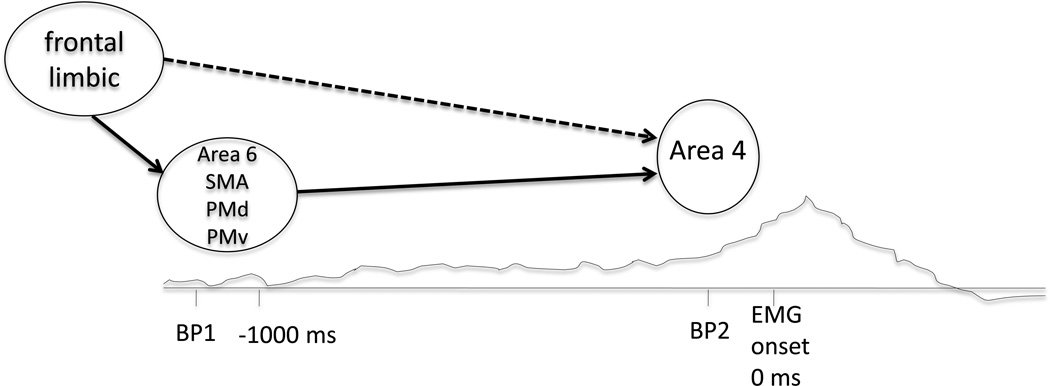
Figure 2.
Localization of the BP1 and BP2. After the drive to move originates from frontal and limbic regions among others, the BP1 is a slowly rising bilateral negativity starting >1 second prior to movement onset. The BP1 reflects activity in bilateral area 6 comprised of SMA, PMd and PMv. The faster rising negativity immediately preceding movement is BP2, reflecting additional activity in contralateral area 4, the primary motor cortex or M1. There are situations, such as tics, in which the drive to move may act on area 4 directly without much involvement of area 6, in which case only the BP2 would be seen prior to movement onset (dashed line). BP = Bereitschaftspotential, SMA = supplementary motor area, PMd = dorsal premotor cortex, PMv = ventral premotor cortex.
The similarity between the BP2 prior to these patients’ tics and the shortened MRCP prior to movements made in response to a stimulus supports the clinical impression that tics occupy a liminal space between the concrete ideas of voluntary and involuntary, and that at least on a physiological level there exists a difference between choosing to act and acting because some signal, internal or external, compelled one to do so. Additionally, the Karp et al. study further disproves the BP as a correlate of voluntariness. As has been shown previously, the BP is not required for the presence of volition, since not all healthy subjects with normal volitional movement have BPs prior to their movements (Jahanshahi and Hallett, 2003), and the presence of the BP does not mean that the movements will be experienced as voluntary, as demonstrated in this study.
A difference in the neural processes preceding movement has also been shown in behavioral paradigms in patients with tics. In the case of “Libet’s clock,” subjects use a rotating clock hand on a computer screen to report the time of willing a simple motor act (“W”) and in other trials, the time of actual movement onset (“M”) (Libet et al., 1983). Libet’s original work showed that the brain preparation for movement began well in advance of when subjects would report the conscious intention to move. The sense of volition, therefore, arises “late” in the development of the motor initiation processes. Since the sense of volition must precede movement to have the sense of causality, its timing is likely to be critical. When patients with tics performed normal voluntary movements in a Libet-clock paradigm, they reported times for “W” consistently later (closer to the time of movement onset) than controls, despite reporting times for “M” similar to those of controls (Moretto et al., 2011). This delayed experience of intention correlated with the degree of tic severity, and other factors such as comorbidities and medication usage failed to account for this difference in regression analyses. The delayed experience of movement intention in tic patients, demonstrated here for normal movements such as pressing a computer key, may be a consequence of ongoing tic suppression. If a Libet clock-type experiment were done for only the tics, it might be expected that time “W” might be delayed since the tic is preceded only by the BP2 and not the entire BP (if there is some mechanistic relationship between the mechanisms of the BP and W). Why these patients’ normal movements such as key presses would also be affected is not clear, but is consistent with a role of top-down influences in the theoretical framework for volition. By this reasoning, living with the frequent experience of involuntary urge and compulsory movement may influence the patient’s expectations regarding any movement.
Any neuroimaging of patients with unpredictable rapid movements is inherently fraught with difficulty, and thus much of the neuroimaging work in tics has consisted of structural MRI, metabolic PET, or resting state functional MRI. Differences have been found in amygdalar, hippocampal and caudate volumes, as well as cortical thinning in the somatosensory areas (Fahim et al., 2010, Peterson et al., 2007, Sowell et al., 2008), and correlation has been made between some of these changes and the degree of tic severity (Bloch et al., 2005, Thomalla et al., 2009). The results of these investigations are often difficult to parse into various contributions given the frequent neuropsychiatric comorbidities such as OCD (Ludolph et al., 2008) as well as exposure to neuroleptics and other centrally active medications. In one recent study of 60 adults with TS by Worbe et al, the nature of the tic disorder (simple tics, simple and complex tics, or tics with associated OCDs) predicted the location of cortical thinning (Worbe et al., 2010); as in all studies of structural abnormalities in adults with TS, however, it is difficult to know if these differences are causative or an effect of living with the disorder.
Since some patients describe the tic as “voluntary” and the premonitory sensation as “involuntary,” using temporal-based neuroimaging methods would be helpful in differentiating between these experiences and the subjective sense of volition associated with each, but this typically involves technically difficult signal processing to eliminate head motion-associated artifact. In one analysis of 22 TS patients allowing their tics to freely occur in the scanner, Bohlhalter et al. performed a time-course analysis of maximum BOLD signal intensity at the time of tic onset as well as at two seconds prior to tic onset in order to capture brain activation associated with the urge to tic (Bohlhalter et al., 2006). In these patients, the pattern of brain activation two seconds prior to the onset of patients’ tics highlighted paralimbic and sensory association areas, such as anterior cingulate, insula, supplementary motor area, and parietal operculum. Insular activation was also prominently featured in the [15O]H2O PET study of Lerner et al., in which nine patients with GTS were studied both awake, allowing tics to freely occur, as well as during stage 2 sleep (Lerner et al., 2007) When compared with stage 2 sleep, the state of tic generation was associated with activation in the bilateral insula and cerebellum, as well as putamen, thalamus, SMA and motor cortex. Wang et al also imaged 13 patients with GTS although only with right facial tics; in one condition patients allowed tics to freely occur, in another they voluntarily mimicked the right facial tic at a frequency such as to prevent buildup of any urge, and in another condition healthy volunteers mimicked the right facial tic (Wang et al., 2011) Comparing these conditions, the authors propose that the network of somatosensory and posterior parietal cortices, putamen, amygdala and hippocampus showed greater activation during the first condition and thus were more likely to be associated with urge to move. While these studies have disparate results within the sensory and limbic networks, the divergence in activation patterns may be related to the differing phenomenology, since all tics in Wang et al’s study had the same semiology whereas Bohlhalter et al. and Lerner et al. imaged patients with a variety of tics. Additionally, while Bohlhalter et al. attempted to capture the brain activation associated with urge, Wang and Lerner et al’s studies looked at the brain activation over a period of time, thus capturing urge, tic generation, and tic release. In a meta-analysis of functional MRI activations in other urges (yawning, swallowing, micturition), the neuroanatomy of these urges were found to overlap with activations during urge to tic (from the Bohlhalter et al. study) in the bilateral dorsal anterior cingulate and right insula. (Jackson et al., 2011)
Tics may represent over-learned motor programs, similar to habits, inappropriately selected and rewarded. As movements are repeated frequently over years, automation of the motor program increases the movement’s efficiency to the point that conscious attention to the movement is no longer necessary, as in the example of how we can walk without thinking about the details of stepping. Tics may thus be internally-driven movements, performed in response to an internal stimulus (the urge to move) by-passing area 6 (see Fig. 1), and would therefore be expected to be preceded by the BP2 only as has been shown. The source of this internal signal is not yet clear, but may have its etiology in the paralimbic and sensory association areas found to be activated in the neuroimaging studies of tics. It follows that allowing such a compulsory act to occur would be associated with a very different sense of volition and accompanying confusion regarding whether it was done voluntarily or involuntarily. There may also be a relationship between the simplicity of motor tics and an eventual blurring of consciousness and voluntariness for the movement over time: the simpler the motor tic, the more likely it may be for the motor program to be automated, for the MRCP to be shortened to the BP2 only, and for the movement to be easily executed without even reaching conscious awareness. More complex tics, or actions that require the patient to direct their attention to the tic such as tic suppression, would therefore be more likely to trigger a sense of volition.
Until recently, tics were not treated with any attempt at behavioral modification, whereas behavioral therapy would frequently be part of a treatment regimen in a patient with obsessive-compulsive disorder who excessively washed his hands, for example. This distinction may be an artifact of the reductionist dualism still lingering between psychiatry and neurology, in which an “organic” movement disorder would not be considered amenable to psychotherapy, or it may represent caution on the part of neurologists given that tics were considered psychogenic until the 1960’s when treatment response to antipsychotic medication was first recognized. While early case series of habit reversal therapy reported success in treating individual tics (Azrin and Peterson, 1988), these methods gained greater recognition in the literature in the past ten years. Habit reversal therapy (HRT) comprises training in awareness, relaxation, competing response, motivation and generalization in order to substitute a competing muscle movement for the unwanted behavior such as trichotillomania or stuttering. In one meta-analysis of habit reversal therapy including five studies with a total of 218 patients (children and adults) with TS, the effect size of HRT was 0.78, large with regards to success, and similar to that of other disorders in which it was used (Bate et al., 2011). Interestingly, in the largest randomized controlled trial of HRT in children with TS, as well as the meta-analysis of Bate et al, there is no mention of voluntary or involuntary except that the competing movement with which the patient attempts to replace the tic will be voluntary (Piacentini et al., 2010). Referring to the forward model diagram (Figure 1), the execution of the motor program results in relief of unpleasant internal tension as a higher order effect of movement. This relief may feed back to reward centers in the prefrontal and limbic cortices that can generate the drive to move, and eventually result in a habit-like behavior that can activate the motor system directly at area 4 but with a less strong feed-forward signal that may result in a less robust sense of will or agency. This decreased sense of volition will inform the posterior going forward. If the competing behavior is imbued with the “reward of success,” then it could replace the tic as a favored behavior.
Psychogenic Movement Disorder or Motor Conversion Disorder
While psychogenic weakness, psychogenic paralysis, and psychogenic hyperkinetic movement disorder (PMD) share a common presumed etiology, an important distinction exists between these with regards to the components of volition. In hyperkinetic PMD, patients experience a movement without an associated sense of willing or agency, whereas with psychogenic weakness, patients have an intact sense of willing but are not able to complete the movement. In full psychogenic paralysis, there are claims of normal willing, but no movement and no sense of agency.
In patients with hyperkinetic PMD, the abnormal movements share certain characteristics with volitional movement, but they are experienced by the patient as involuntary (Hallett, 2007, Hallett, 2010). If a patient with ongoing psychogenic movements is asked to perform a demanding voluntary task, the psychogenic movements will typically disappear for the duration of the task (distractibility). In the case of psychogenic tremor, if the voluntary task is performed at different frequencies, such as tapping with the unaffected hand, the involuntary tremor will typically assume the rhythm of the tapping (entrainment) or a harmonic of the rhythm of tapping, or otherwise change in frequency (Brown and Thompson, 2001, Deuschl et al., 1998, McAuley and Rothwell, 2004, Zeuner et al., 2003). Similarly, a large amplitude movement in the unaffected hand will typically cause an ongoing psychogenic movement to pause briefly (Kumru et al., 2004). In contrast, a patient with Parkinson disease, for example, will continue to have tremor regardless of any other motor task he or she performs; in such a patient, the motor task will be interrupted by the abnormal movements, rather than the abnormal movements interrupted by the motor task. Additionally, electroencephalographic investigations have shown the presence of a normal looking BP prior to the onset of psychogenic movements indicating involvement of the premotor cortex (Terada et al., 1995, Toro and Torres, 1986). In this sense the involuntary abnormal movements in PMD cannot be differentiated on an electrophysiological basis from the patient’s normal, voluntary movements. These studies show a sharing of psychogenic and voluntary mechanisms of the proximal machinery for movement, but the more distal generation is less well understood. Moreover, there is no sense of “normal” willing of movement in the psychogenic situation.
Behavioral studies that attempt to isolate the neural processes surrounding volition have been explored in this patient population. When the Libet clock paradigm was evaluated in patients with psychogenic tremor, the time of intention to move, “W”, was significantly later (closer to movement onset) in patients compared to healthy controls (Edwards et al., 2011). The same result was found in the unaffected arms of those patients with unilateral psychogenic tremor as well as those with bilateral psychogenic tremor, indicating that even “normal” movements were associated with a diminished sense of voluntariness. Similarly, changes were seen in “normal” movements in PMD patients using the paradigm of intentional binding. In this paradigm, subjects use a Libet-type clock to report the time they performed an action, such as a key press, or observed its effect, such as an auditory tone. When normal subjects perceive themselves to be agent responsible for the effect, they judge actions to occur later and effects to occur earlier when coupled compared to baseline trials consisting of only actions or of only tones (Haggard et al., 2002). In the case of an involuntary movement such as a key press passively induced by TMS, the reverse effect was found: actions were judged to occur earlier and effects to occur later when coupled compared to the baseline trials (Haggard and Clark, 2003). When this task was explored in patients with hyperkinetic PMD making normal movements (key presses), patients demonstrated less binding than that seen in healthy controls (Kranick et al., in press). Comparing twenty patients with motor conversion with a variety of hyperkinetic movements to twenty matched controls, patients reported times of the key presses and resultant tones closer to their actual times than in controls, while controls exhibited the action-effect binding previously demonstrated in this paradigm. These results imply a reduced effect of agency in patients with PMD, even for their “normal” voluntary movements.
Structural analyses in patients with hyperkinetic PMD and similar disorders have suggested possible abnormalities in the limbic areas. In a study of 20 patients with psychogenic non-epileptic seizures, Labate et al. show abnormally low gray matter volumes in the bilateral cerebellum, right precentral gyrus, right middle frontal gyrus, anterior cingulate and SMA, and abnormal cortical thinning in the right precentral gyrus, right superior frontal gyrus, right precuneus, and right paracentral gyrus (Labate et al., 2012). A similar study using VBM and cortical thickness analysis in 16 patients with hyperkinetic PMD found smaller gray matter volumes in the left amygdala, right hippocampus and bilateral nucleus accumbens, increased cortical thickness in the left precentral gyrus, right rostral middle frontal gyrus and precalcarine gyrus and decreased cortical thickness in the left cingulate cortex and right anterior cingulate (Czarnecki et al., 2012). These analyses point to important differences between these patients and healthy controls that are not detected on a standard MRI but may be clinically meaningful either with regards to the etiology of the disease or the effect of living with abnormal movements.
Early neuroimaging studies in psychogenic paralysis demonstrated a network of brain areas activated in these patients not limited to the motor areas but also including limbic structures such as the prefrontal cortex and the anterior cingulate (Marshall et al., 1997, Spence et al., 2000, Tiihonen et al., 1995, Vuilleumier et al., 2001). The pattern of activation seen in these studies has led some authors to theorize that sensorimotor areas may be actively inhibited by limbic structures, preventing the limb from moving normally in psychogenic paralysis. While limbic inhibition of the motor network may explain the absence of movement in psychogenic paralysis, limbic facilitation might explain psychogenic hyperkinetic movements.
In a series of three fMRI experiments, Voon et al have built a framework for how abnormal activation and connectivity between limbic and sensorimotor areas might explain not only the reduced sense of volition experienced by PMD patients but also how their abnormal movements are generated. The first of these studies represents the first published attempt to study patients with hyperkinetic motor conversion during their movements, as eight patients with conversion tremor were asked to either “trigger” their conversion tremor or to voluntarily mimic their tremor during different blocks of the study (Voon et al., 2010). During the blocks of conversion tremor, patients showed not only decreased activation in the right temporoparietal junction but also decreased connectivity between this region, the sensorimotor areas, and limbic regions such as the ventral anterior cingulate and ventral striatum. In the next study, 16 patients with conversion disorder of varying hyperkinetic movement type, as well as matched controls, were presented standardized emotional stimuli during fMRI (Voon et al., 2010). While the patients showed the same level of amygdala activation for the faces regardless of emotional valence, this activation failed to habituate as fearful faces were shown repeatedly, as it did in controls. Greater functional connectivity was also seen in patients between the right amygdala and right supplementary motor area during fearful or happy stimuli compared to neutral stimuli. In the third fMRI study, 11 patients with motor conversion and matched controls were asked to make either internally and externally generated movements (Voon et al., 2011). During both internally and externally generated movements, patients compared to controls had lower activation in areas implicated in motor initiation such as the left supplementary motor area (SMA), and higher activation in areas implicated in assigning emotional salience, such as the right amygdala, left anterior insula, and bilateral posterior cingulate. The left SMA in conversion patients also showed decreased functional connectivity with the bilateral dorsolateral prefrontal cortices (DLPFC) during internally generated action compared to externally generated action; given the role hypothesized for the DLPFC in guidance of action selection, the authors suggest that conversion patients may have an impairment in top-down motor control when selecting an action. Taken together, the results of these three studies suggest that in PMD or motor conversion, in the context of emotionally valent stimuli, there is abnormal increased activity between the amygdala and the SMA, hypoactivity in regions involved in motor selection and hyperactivity in those engaged in assigning salience. Without appropriate top-down control from the DLPFC, this may result in selection of an inappropriate action. The proprioceptive feedback of this inappropriate movement would not match with the intended motor program, thus generating a sense of mismatch as occurs with involuntary movement.
The term “psychogenic” is deceptively simple in its assumption of a psychological etiology for abnormal movements in these patients, as is the term “conversion.” The physiologic mechanism by which psychic distress could be converted into neurological symptoms has yet to be determined. While there are many patients with motor conversion disorder who have clear stressors triggering the onset or exacerbation of abnormal movements, in whom comorbidities of depression and anxiety can be diagnosed and treated, this is not always the case. A significant subpopulation of patients without clear psychopathology are now increasingly recognized and described in the literature (Kranick et al., 2011, Stone and Edwards, 2011), which is of clinical importance as these patients are likely the most refractory to traditional treatment approaches. These patients frequently demonstrate no secondary gain; instead, their lives have more likely been devastated by the ongoing involuntary movements and the ensuing disability. Taking Freudian logic to its ultimate conclusion, these patients may represent a “full conversion,” such that no psychological symptomatology can be found because any underlying psychic distress has been successfully converted into abnormal movements. Alternatively, there may be multiple disorders with varying degrees of discernable psychological contribution encompassed within the spectrum of PMD. Differentiating between these possibilities will require greater understanding of how these movements are generated in the brain and how the sense of volition for these movements is subverted.
Given the difficulty in convincing PMD patients to recognize a psychological basis for their abnormal movements, especially in those who have no clear associated psychopathology or psychiatric diagnosis, the question arises whether “symptomatic” therapy could work for PMD. Just as the question of voluntary and involuntary is side-stepped when using habit reversal training for tics, similar methods of treatment would attempt to reduce the patient’s abnormal movements without addressing the etiology or mechanism. This is already done to some extent by using physical therapy independent of psychological treatment in patients with PMD. Czarnecki et al. describe a successful protocol of “motor-reprogramming” carried out in 60 patients with PMD (Czarnecki et al., 2012). The rehabilitation strategy was described to patients in “operational terms,” such as a “’disconnect’ between the patient’s normal brain motor program and the normal nerves/muscles used to carry out the movement,” with no attempt “to explain how the ‘disconnect’ initially occurred.” Despite a median duration of 17.5 months of abnormal movements at the time of entering the study, approximately 70% were significantly improved at the end of one week in the rehabilitation protocol, and in those who completed follow up questionnaires or interviews (80% of the treatment group) at a median of 25 months after treatment, 60% had significant sustained improvement. Critics of this approach will point out that if underlying psychological issues such as childhood trauma are not addressed, the psychic distress will manifest time and again via other somatic manifestations. In the only study of antidepressant treatment in patients with PMD, two subgroups were identified: ten patients with primary conversion disorder, all of whom had current or previous depressive or anxiety disorders, of whom eight had marked improvement and seven had complete remission; and five patients with primary hypochondriasis, somatization disorder, or probable factitious disorder/malingering, of whom none improved (Voon and Lang 2005). This treatment-refractory subgroup of patients, in whom antidepressants are not effective and in whom symptomatic therapy would only be temporarily beneficial given the predisposition for further somatization, represents the greatest treatment challenge in PMD or motor conversion.
What does the study of psychogenic movement disorders teach us about the nature of volition? These patients have excess movements, often distracting and disabling, but these appear to share certain characteristics with volitional movements. What sets PMD patients clinically apart from most patients with tics, however, is the denial of any sense of volition for the movements; they are not performed a compulsory basis, but rather the PMD patient states that the movements “just happen” without any warning or opportunity for suppression. The PMD patient often has a strong sense that their disorder is an organic disease, such as a brain tumor, and not psychological. This might be considered a Bayesian prior influencing the perceptual integrator (Fig. 1). The structural and functional neuroimaging studies suggest a network of abnormal inputs from the limbic areas that may trigger movement (or block it in the case of paralysis), not producing a normal feedforward signal. With the resultant mismatch between the actual movement genesis, feedforward signal and the prior expectation about how movements should be willed, there would be a loss of the sense of both willing and agency. Hypoactivation of the right temporoparietal junction with psychogenic movements is consistent with this idea.
Passivity Phenomena in Schizophrenia
Among the delusions and hallucinations experienced by patients with schizophrenia, one which stands as a hallmark of this disease is the delusion of control, a type of passivity phenomena. Patients who experience delusions of control make seemingly normal movements but claim that these are the product of some other agent, and not the result of their own will. Unlike tics and PMD, these patients do not have abnormal unwanted movements; the lack of volition that the patient feels is for the normal actions of daily life. These movements serve the patient’s purposes; the same goals are met with the same precision and efficiency as is seen in healthy controls, indicating that the process of movement generation is intact although the sense of volition is lacking.
Since there is no need to explain the generation of abnormal movements in the case of these patients, researchers have considered whether a defect in the perceptual integrator (as in Fig. 1) alone could sufficiently account for passivity phenomena (Frith et al., 2000). Thus neuroimaging studies in patients with passivity phenomena are of particular interest with regards to the potential for localizing the perceptual integrator in the brain. A [15O]H2O PET study by Spence et al showed hyperactivation in the right inferior parietal lobule (Brodmann area 40) and the cingulate gyrus in schizophrenic patients with passivity during free action selection compared to schizophrenic patients without this symptom (Spence et al., 1997). Studies investigating brain activity during mismatch between a subject’s action and distorted feedback have often suggested that such mismatch detection is associated with increased activity somewhere in the right parietal lobe, although whether this is the temporoparietal junction, the inferior parietal lobule, or even the posterior parietal cortex has varied between studies; additionally, other regions such as the anterior insula, medial and dorsal prefrontal cortex, supplementary motor area, and the cerebellum have also been implicated (see (David et al., 2008) for review). The temptation to consider the perceptual integrator to be thusly localized must be tempered somewhat by the difficulty in comparing coordinates between studies, but increasing evidence would argue that the right parietal cortex carries significant responsibility for mismatch detection (Sperduti et al., 2011).
While the perceptual integrator has not yet been located conclusively in the brain, nor has it been possible so far to track generation of the motor program or efference copy by electrophysiology, there is substantial evidence from research that the brain is constantly comparing internal predictions to external feedback as indicated in Fig. 1. This is evident in our ability to track moving objects without perceiving the entire visual field as moving: during smooth pursuit, images of a stationary environment inevitably project to the retina at the same speed as eye rotation. How is it that we do not then see the world moving around us? It has been demonstrated that by comparing the rate that the images slip over the retina with the amount of image motion predicted by the motor command to move the eyes, our brains interpret the match of these signals as evidence that we are tracking a moving object (Haarmeier et al., 2001). It would follow that schizophrenic patients with passivity are unable to attribute their own actions to themselves because of missing efference copy or defective comparison to proprioceptive feedback of normal movement. Studies have shown an impaired ability to distinguish between externally- and self-produced actions in schizophrenic patients: schizophrenic patients recognize self-induced movement as ticklish, unlike healthy controls who are typically unable to tickle themselves (Blakemore et al., 2000). Similarly, schizophrenic patients have shown an impaired ability to recognize retinal image motion from smooth pursuit as self-generated (Lindner et al., 2005).
In multiple studies using paradigms of modulated feedback for actions, however, schizophrenic patients showed a tendency to over-attribute events to themselves, claiming a sense of volition for actions even when mismatched feedback would cause a healthy control subject to feel that the action was externally driven (Franck et al., 2001, Haggard et al., 2003, Knoblich et al., 2004). In a perceptual adaptation paradigm in 20 schizophrenic patients with passivity phenomena and matched control subjects, subjects made pointing movements with their right arm, hidden from view, and used a mouse in their left hand to point a cursor in the direction that they thought their right hand was pointing (perceived pointing direction or PPD). (Synofzik et al., 2010). After receiving consistently manipulated visual feedback, both controls and patients showed adaptation by reporting the PPD to be consistent with the rotation of feedback, and by overshooting when reaching for a target in the opposite direction. The patients, however, reported the PPD after adaptation to be shifted by almost twice as much as in controls, and demonstrated significantly greater variability in their PPD reports; in this way the patients seem to be more reliant on external cues for making sensory judgments, and less able to trust internal predictions. The variability of the schizophrenic patients’ PPD judgments correlated with their impairment in differentiating external from internal action in a separate experiment, as well as with the degree to which they experienced delusions of control. Importantly, a similar paradigm of sensory prediction has been investigated in subjects who do not meet criteria for schizophrenia but demonstrate higher levels of “delusion-like ideas” on schizotypy scales (Teufel et al., 2010). In this study subjects with greater tendency towards delusional ideation were less able to predict the sensory consequences of self-generated movement, again reinforcing the idea that schizophrenics as well as those with schizotypal-type symptoms have a greater dependency on external cues when making judgments about volition, and this effect cannot be explained by neuroleptic medication.
Using the intentional binding paradigm with a probabilistic component, Voss et al studied 24 schizophrenic subjects and matched controls (Voss et al., 2010). Subjects used a rotating clock on the computer screen to report the time of key presses, which resulted in a tone in 50% of trials in two blocks and 75% of trials in two blocks. Similar to previous studies in which patients with schizophrenia over-attributed actions to themselves despite considerable mismatch, schizophrenic patients in the Voss et al study showed even greater intentional binding than controls. When the intentional binding paradigm has been modulated with this probabilistic component previously, healthy controls show a greater predictive contribution to binding in the 75% condition than in the 50% condition: when the key press has a 75% chance of being followed by a tone, the subject will demonstrate intentional binding even on the trials when the tone is absent, but not if the key press has only a 50% chance of being followed by a tone (Moore and Haggard, 2008). This greater predictive contribution to binding was shown again in the controls in this study, whereas the patients with schizophrenia demonstrated the same degree of binding in the 50% and 75% conditions, therefore showing less reliance on internal prediction models. In comparison to the control subjects, the patients with schizophrenia were much less likely to demonstrate binding in a single trial when the tone did not occur, emphasizing the patients’ reliance on external sensory cues and retrospective inference in making judgments regarding volition.
That schizophrenic patients with passivity do not simply under-attribute the effects of their actions in behavioral studies reinforces the significance of feedforward and feedback signals in the theoretical framework of volition. These patients have fixed delusions in which their actions are being willed by some other agent, but they also differ from healthy controls in their inability to rely on internal prediction models and their increased dependence on external sensory cues.(Moore and Fletcher, 2012) As a result of this body of research, it has become increasingly clear that both internal cues (such as efference copy and mismatch detection) as well as external cues (such as the effect of a given action) are required for the sense of agency. When applied to schizophrenia, it has been proposed that these patients rely excessively on external feedback (in Fig.1, effects of the movement occurring) with less attention to internal predictive models (areas 4 and 6). The disparity between the weight given to internal and external cues may result in a sense of mismatch, with attenuation in the sense of will and agency. The decreased sense of agency informs the posterior, which becomes the prior going forward, and the sense of reduced agency may also contribute to a greater weight being placed on external cues going forward. The increased weight of external cues may bias the belief system to the sense of external control.
Alien Hand Syndrome
The term “alien hand” encompasses multiple different types of abnormal movements with varying pathology which are grouped together by the patient’s lack of self-agency for movements made by one of their arms. While quite rare in clinical neurology, the phenomenon of alien hand syndrome (AHS) is easily recognized by the public due to Peter Sellers’ character in the movie Dr. Strangelove, whose hemiparetic arm works as cross-purposes with the rest of his body. Seeming to have a “mind of its own,” the affected arm in this exaggerated cinematic example even belies the character’s latent Nazi sympathies. While all of the subtypes of alien hand phenomena lack self-agency for the affected limb, of particular interest to the study of volition are those patients in whom, like Dr. Strangelove, the affected arm actually works in opposition to the unaffected arm, termed diagonistic dyspraxia (two agonists). This anarchical movement is more commonly seen in AHS following lesions of the corpus callosum and/or anteromedial frontal cortex, with or without unilateral or bilateral SMA involvement. AHS in the context of corticobasal degeneration (CBD) will be discussed in the next section.
In patients with AHS due to callosotomy or anterior cerebral artery infarction, the theme of opposition between the arms pervades the reported cases, in which the left hand might undo buttons as the right hand tries to button the shirt, or the left hand might attempt to take the pencil out of the right hand during writing (Biran and Chatterjee, 2004). The right hand is more often affected in AHS in CBD, whereas the left hand is more often affected in AHS due to damage to the corpus callosum.
Neurophysiological and imaging investigations in patients with AHS have revealed differences in how the brain prepares for movement in the affected hemisphere. Tanaka et al reported a case of AHS in which a man with callosal damage had anarchical movements of the left hand. In this patient the Bereitschaftspotential (BP) over the right hemisphere was observed only when the patient initiated voluntary activity with the right hand and not when the left hand moved independently (Tanaka et al., 1990). In one patient with AHS after a right parietal stroke, fMRI of left hand movements revealed that alien hand movements involved a selective activation of contralateral primary motor cortex, while voluntary movements activated a distributed network of not only the contralateral motor and premotor cortices but also the left inferior frontal gyrus (Assal et al., 2007). These physiological findings seem to argue that the alien movement arises from relatively isolated activity of the primary motor cortex, but when this happens, it will not be accompanied by a sense of volition. This mode of activation, without premotor cortex, is consistent with a lack of feedforward signaling, without which there can be no sense of will or agency for movement and thus it will be interpreted as involuntary.
While these studies address the lack of volition the patient feels for the “alien” hand, they do not explain why the affected hand would work specifically to oppose the movements of the unaffected hand. It is as if the hand is trying to reverse a change that “it did not will.” The abnormal, diagonistic movements of the affected hand do not usually occur spontaneously but rather in the context of planned, goal-directed movement in the unaffected hand. Frith et al have proposed that damage to the SMA in these patients removes an inhibitory influence that prevents inappropriate reaching for and grasping of objects in the environment (Frith et al., 2000). If movement is actually precipitated by lack of inhibition, then the deficiency of SMA activity could be responsible both the movement genesis and a failure of feedforward signals, without which there will be no sense of will for the movement and no self-agency since there is no match for the perceptual feedback.
Utilization Behavior
AHS may also occur in the context of corticobasal degeneration, a rare neurodegenerative syndrome of accumulation of the protein tau, affecting in particular the parietal and posterior frontal cortices. In patients with CBD with AHS, there is a greater preponderance of complex reflexive movements, such as grasping or inability to release, as well as mirror movements, tendency to levitate or “drift off,” or assume odd postures. Utilization behavior is also seen in these patients. As described by Lhermitte, utilization behavior (UB) refers to automatic-appearing movements involving objects that are appropriate for the object but not for the context.(Lhermitte, 1983) For an example of UB, if a neurologist places a toothbrush in front of a patient during an exam without instructions, it would be contextually inappropriate for the patient to pick it up and start brushing her teeth. These behaviors, along with inappropriate imitation of other’s gestures and movements as seen in imitation behavior (IB), may be part of a larger syndrome of environmental dependency, and are usually found in patients with frontal lobe lesions such as frontotemporal dementia (FTD). Various theories have been proposed for how such “automatic” behavior might be “released” from normal motor control, but this might be because of excessive influence of sensory triggering of movement compared with internal triggering due to the frontal degeneration [for comprehensive discussion, see (Archibald et al., 2001)]. While UB may help differentiate between FTD and other dementias, it is still quite rare and there are relatively few reports in the neurological literature. (Ghosh and Dutt, 2010) Thus there is little description of how these movements contrast with the patient’s will or agency, other than the fact that these movements may persist despite instructions not to touch the object. For this reason they are described as “involuntary,” “nonpurposeful,” or “outside of the patient’s control,” similar to a reflex or an automatic movement made in response to external stimuli. (Archibald et al., 2001)
Other Syndromes Affecting Volition
While frontal lobe lesions may result in abnormal behavior such as anarchic hand movements or utilization behavior, damage to the frontal lobes may also manifest as a lack of spontaneous or normal behavior. This may be characterized by apathy or abulia, or in its most profound form, akinetic mutism. Akinetic mutism describes a syndrome in which patients show a drastic reduction in motor output, including automatic movements, gestures and speech, without weakness or other causative factors. While this syndrome has been described in association with strokes or other lesions in the frontal lobes, basal ganglia, and thalamus, it has also been used to describe behavior in Parkinson disease, Alzheimer’s disease, FTD, Creutzfeldt-Jakob disease, and catatonic schizophrenia. (Nagaratnam et al., 2004) Thus the neuroanatomical structures implicated vary widely between reports. In some cases, damage to the mesial frontal lobes including the cingulate and SMA have been implicated.(Meador et al., 1986) These disorders are all related to the deficient internal triggering of movement that arises from various systems in the frontal lobe. The basal ganglia support frontal lobe function so it is consistent that basal ganglia disorders will also be characterized by difficulty in the self-initiation of movement.
Syndromes of Hyper-Volition
In comparison to the cases described above, generally connected by decreased sense of volition in the patients involved, there are also less frequent instances in which neurology patients will claim voluntariness inappropriately, both when movements are clearly involuntary as well as when there is no movement.
Huntington Disease
It is our experience, as well as that of many neurologists, that patients with chorea early in the course of their disease will claim voluntariness for involuntary movements when these are relatively infrequent. When asked if they were making a movement on purpose or if a movement happened to them, they will often describe the movement in automatic terms, such as “I was just stretching” or “I was scratching my head.” While this phenomenon has not specifically been studied, there is evidence for several potential contributing mechanisms in over-attribution of will in patients with Huntington disease (HD).
Numerous studies have suggested differences in movement in pre-manifest HD patients as well as in voluntary (non-choreiform) movement in affected HD patients. Movement-related potentials have been found to be abnormally reduced in slope and peak in HD patients, indicating defects in motor preparation (Johnson et al., 2001). Sensory feedback from the movement itself is also impaired in HD: somatosensory evoked potentials (SSEPs) in patients with HD are typically abnormal despite a lack of sensory complaints. Frequently the abnormality seen on SSEPs is reduced amplitude of the early components from the parietal and frontal cortices, likely reflecting abnormal transmission from the thalamus to the sensory cortex (Abbruzzese and Berardelli, 2003). Voluntary movement in HD patients relatively unaffected by chorea has been characterized as inconsistent and inefficient (Phillips et al., 1996), possibly due to reliance on external visual cues (Georgiou et al., 1995), or difficulty using internal predictive models to cue movement (Bradshaw et al., 1992).
These behavioral findings are strikingly similar to what is seen in schizophrenia patients with delusions of control, who paradoxically over-attribute actions to themselves. In pre-manifest HD patients, the Bayesian prior or working hypothesis regarding volition would be that the patient has will and agency for their movements, since in this neurodegenerative condition they have lived for years without overt motor symptoms. There may be a diminished influence of perceptual feedback, evidenced by abnormalities in SSEPs. Chorea likely arises as the result of abnormal noise in the movement execution system. Integrating the demonstrated over-reliance on external visual cues and under-reliance on internal predictive models, the patient would therefore be less likely to challenge the prior, despite the lack of feedforward signal, until involuntary movements become frequent and more obvious.
Anosognosia
In anosognosia, usually occurring after stroke or another brain injury, the patient does not recognize or appreciate the severity of a neurological deficit. This occurs most commonly as anosognosia for hemiplegia after a stroke in the right hemisphere. When asked to try to move the left arm, patients will insist that they are moving it; when the flaccid arm is brought into the patient’s view by the examiner, they will deny that the arm is theirs. The possible manifestations of anosognosia are myriad: the patient may demonstrate varying degrees of denial of ownership of the affected arm, including attributing to others (somatoparaphrenia), or recognize the deficit but deny its importance, or refuse to acknowledge weakness and confabulate regarding movement. The patient may perform tasks as if both hands were available, while others may take a unimanual approach to such tasks, indicating an implicit knowledge of the hemiparesis (Cocchini et al., 2010).
Once considered to be a form of psychological denial for the patient’s weakness (Weinstein and Kahn, 1955), or a consequence of cognitive deficits (Levine, 1990), the understanding of anosognosia has developed considerably with the proposal of the forward model, considering anosognosia as a deficit of motor planning (Frith et al., 2000). Heilmann et al considered anosognosia a “motor intentional deficit,” with the defect at the level of generation of the motor program (Heilman et al., 1998). By this theory, even if the perceptual integrator is intact, if no motor program is generated then the patient considers the movement to have been completed successfully. By contrast, Berti et al proposed that the deficit in anosognosia affects the perceptual integrator, with intact generation of the motor program (Berti et al., 2005). Using a lesion mapping study to determine which brain areas were specifically lesioned in those patients with denial of their deficits, they found denial to be associated with network of lesions in the motor, premotor, and sensory cortices, with some areas of motor planning relatively spared. Further investigating the perceptual integrator and mismatch detection in anosognosia, Fotopoulou et al. developed a rubber hand paradigm to determine the relative contributions of motor planning and sensory feedback to the assertion of being able to move the limb. Patients were significantly more likely than controls to ignore the visual feedback of a motionless hand and claim they moved it when they intended to do so than when they expected the experimenter to move the hand, indicating that these patients are far more reliant on internal predictive models than on sensory feedback (Fotopoulou et al., 2008). The presence of intact motor programs was further confirmed by Garbarini et al, who used a bimanual interference task in patients with anosognosia (Garbarini et al., 2012). While these patients as predicted did not make any drawing with their left (paretic) hands, by asking the patients to draw circles in the left hand and straight lines in the right, the lines of the right hand took on an oval shape, as is seen in controls. In this way anosognosia was shown to be more than just a cognitive reaction to a motor deficit, since the motor program, still intact, can compete with a motor program in the unaffected arm as it would normally.
The anosognostic patient has an intact sense of will when attempting to move the arm, but they also claim agency for movement after no movement is produced. Can the intact motor program, as demonstrated in the studies above, be sufficient for the generation of the sense of agency? Such a model would seem to negate the powerful contribution from sensory input and the perceptual integrator when making judgments regarding agency. These patients are particularly reliant on internal predictive models, as opposed to the dependence on external cues seen in schizophrenic patients with passivity or patients with early chorea. As functional networks supporting movement are constantly updating inferential models based on incoming information, patients may become more internally- or externally-driven depending on how their brains are changing with regards to their environment. After a stroke, if the sensory cortex sustains damage, the weight given to various inputs may change, and greater reliance may rest on internal predictive models. By this reasoning, the patient’s lack of sensory feedback may not be given the same importance it would previously, allowing the patient’s prior of being able to effectively complete movements as willed to remain unchallenged.
Conclusion
How do neurological patients inform our understanding of volition? A spectrum of pathology exists in which patients may experience a disordered sense of will or agency for otherwise volitional-appearing movement, or a sense of inappropriate volition for involuntary or absent movement. As opposed to a “lesional” model, in which an infarct of the right parietal cortex, for example, might give rise to a defect in the perceptual integrator and thus movement would be experienced as unexpected and involuntary, disorders of volition seem to have a strikingly multi-level and multi-dimensional pathophysiology. As such, our judgments regarding volition represent our past: patients with neurodevelopmental disorders such as schizophrenia have a prior that represents a lifelong history of unexpected outcomes, and are more reliant on external cues in volitional determinations; patients with neurodegenerative disorders such as early HD have a prior that represents decades of successful motor control and are more likely to attribute action to themselves even when it is involuntary. Our priors in these judgments are changing constantly based on up-to-date information regarding our bodies and our environment, and thus the patient with anosognosia may give greater influence to non-sensory inputs and claim agency inappropriately for movement after some inputs lose their reliability due to stroke. Conversely, a “pathological” prior may develop de novo in patients when excessive salience is assigned to random occurrences (Edwards et al., 2012), and hyper-connectivity between the limbic system and the SMA may explain both inappropriate action selection and an over-reliance on the prior of these actions as involuntary in patients with PMD. Future studies are needed that will not only manipulate one facet of the experience of volition, such as the external cue of timing or of visual feedback, but also investigate other cues, both internal and external, and the role of prior beliefs in the sense of volition. Taken together, the information derived from patients is highly informative about the normal processes that make up the sense of volition.
Table 1.
Neurological Syndromes of Decreased Volition
| Neurological disorder | Will? | Agency? | Semiology |
|---|---|---|---|
| Tics/Tourette Syndrome | Some patients feel their tics are willed, but in response to an urge | Patients generally acknowledge that they are responsible for the movement of the tic | Varies; some tics are simple movements (shrugging, coughing); others are more complex and may appear goal-directed or like compulsions |
| Psychogenic hyperkinetic movement disorder | Patients deny willing the movements | Patients deny agency for the movements | Typically non-goal directed, abnormal movements, often quite disabling |
| Psychogenic weakness | Patients feel that they are willing movement | Patients feel agency for the incomplete movement | Movement occurs but is incomplete |
| Psychogenic paralysis | Patients feel that they are willing movement | No agency for movement | No movement occurs |
| Schizophrenia with passivity phenomena | Patients deny willing movements | Patients deny self-agency for the movements and believe another agent is controlling them | Normal movements |
| Alien Hand Syndrome | Patients deny willing movements | Patients deny self-agency for the movements; the hand seems to have a “mind of its own” | Often movements are at odds with the voluntary movements of the unaffected hand |
Table 2.
Neurological Syndromes of Hyper-Volition
| Neurological Disorder | Will? | Agency? | Semiology |
|---|---|---|---|
| Early Huntington’s Disease | Patients often claim that they were making an automatic-type movement, for which less sense of will would be expected | Patients claim agency for the movements | Early, subtle, choreiform movements that are eventually recognized as involuntary |
| Anosognosia | Patients feel that they are willing a movement in the plegic arm | Patients claim agency for movement | No movement occurs |
Acknowledgments
This research was supported by the NINDS Intramural Program
Footnotes
Conflict of Interest Statement: The authors have no conflict of interest (financial or otherwise) to report as it pertains to the present work.
REFERENCES
- Abbruzzese G, Berardelli A. Sensorimotor integration in movement disorders. Mov Disord. 2003 Mar;18(3):231–240. doi: 10.1002/mds.10327. [DOI] [PubMed] [Google Scholar]
- Archibald SJ, Mateer CA, Kerns KA. Utilization behavior: clinical manifestations and neurological mechanisms. Neuropsychol Rev. 2001 Sep;11(3):117–130. doi: 10.1023/a:1016673807158. [DOI] [PubMed] [Google Scholar]
- Assal F, Schwartz S, Vuilleumier P. Moving with or without will: functional neural correlates of alien hand syndrome. Ann Neurol. 2007 Sep;62(3):301–306. doi: 10.1002/ana.21173. [DOI] [PubMed] [Google Scholar]
- Azrin NH, Peterson AL. Habit reversal for the treatment of Tourette syndrome. Behav Res Ther. 1988;26(4):347–351. doi: 10.1016/0005-7967(88)90089-7. [DOI] [PubMed] [Google Scholar]
- Bate KS, Malouff JM, Thorsteinsson ET, Bhullar N. The efficacy of habit reversal therapy for tics, habit disorders, and stuttering: a meta-analytic review. Clin Psychol Rev. 2011 Jul;31(5):865–871. doi: 10.1016/j.cpr.2011.03.013. [DOI] [PubMed] [Google Scholar]
- Berti A, Bottini G, Gandola M, Pia L, Smania N, Stracciari A, et al. Shared cortical anatomy for motor awareness and motor control. Science. 2005 Jul 15;309(5733):488–491. doi: 10.1126/science.1110625. [DOI] [PubMed] [Google Scholar]
- Biran I, Chatterjee A. Alien hand syndrome. Arch Neurol. 2004 Feb;61(2):292–294. doi: 10.1001/archneur.61.2.292. [DOI] [PubMed] [Google Scholar]
- Blakemore SJ, Smith J, Steel R, Johnstone CE, Frith CD. The perception of self-produced sensory stimuli in patients with auditory hallucinations and passivity experiences: evidence for a breakdown in self-monitoring. Psychol Med. 2000 Sep;30(5):1131–1139. doi: 10.1017/s0033291799002676. [DOI] [PubMed] [Google Scholar]
- Blakemore SJ, Wolpert DM, Frith CD. Abnormalities in the awareness of action. Trends Cogn Sci. 2002 Jun 1;6(6):237–242. doi: 10.1016/s1364-6613(02)01907-1. [DOI] [PubMed] [Google Scholar]
- Bloch MH, Leckman JF, Zhu H, Peterson BS. Caudate volumes in childhood predict symptom severity in adults with Tourette syndrome. Neurology. 2005 Oct 25;65(8):1253–1258. doi: 10.1212/01.wnl.0000180957.98702.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohlhalter S, Goldfine A, Matteson S, Garraux G, Hanakawa T, Kansaku K, et al. Neural correlates of tic generation in Tourette syndrome: an event-related functional MRI study. Brain. 2006 Aug;129(Pt 8):2029–2037. doi: 10.1093/brain/awl050. [DOI] [PubMed] [Google Scholar]
- Bradshaw JL, Phillips JG, Dennis C, Mattingley JB, Andrewes D, Chiu E, et al. Initiation and execution of movement sequences in those suffering from and at-risk of developing Huntington's disease. J Clin Exp Neuropsychol. 1992 Mar;14(2):179–192. doi: 10.1080/01688639208402822. [DOI] [PubMed] [Google Scholar]
- Brown P, Thompson PD. Electrophysiological aids to the diagnosis of psychogenic jerks, spasms, and tremor. Mov Disord. 2001 Jul;16(4):595–599. doi: 10.1002/mds.1145. [DOI] [PubMed] [Google Scholar]
- Cocchini G, Beschin N, Fotopoulou A, Della Sala S. Explicit and implicit anosognosia or upper limb motor impairment. Neuropsychologia. 2010 Apr;48(5):1489–1494. doi: 10.1016/j.neuropsychologia.2010.01.019. [DOI] [PubMed] [Google Scholar]
- Czarnecki K, Thompson JM, Seime R, Geda YE, Duffy JR, Ahlskog JE. Functional movement disorders: successful treatment with a physical therapy rehabilitation protocol. Parkinsonism Relat Disord. 2012 Mar;18(3):247–251. doi: 10.1016/j.parkreldis.2011.10.011. [DOI] [PubMed] [Google Scholar]
- David N, Newen A, Vogeley K. The "sense of agency" and its underlying cognitive and neural mechanisms. Conscious Cogn. 2008 Jun;17(2):523–534. doi: 10.1016/j.concog.2008.03.004. [DOI] [PubMed] [Google Scholar]
- Deuschl G, Koster B, Lucking CH, Scheidt C. Diagnostic and pathophysiological aspects of psychogenic tremors. Mov Disord. 1998 Mar;13(2):294–302. doi: 10.1002/mds.870130216. [DOI] [PubMed] [Google Scholar]
- Edwards MJ, Adams RA, Brown H, Parees I, Friston KJ. A Bayesian account of 'hysteria'. Brain. 2012 May 28; doi: 10.1093/brain/aws129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards MJ, Moretto G, Schwingenschuh P, Katschnig P, Bhatia KP, Haggard P. Abnormal sense of intention preceding voluntary movement in patients with psychogenic tremor. Neuropsychologia. 2011 Jul;49(9):2791–2793. doi: 10.1016/j.neuropsychologia.2011.05.021. [DOI] [PubMed] [Google Scholar]
- Ernst MO, Banks MS. Humans integrate visual and haptic information in a statistically optimal fashion. Nature. 2002 Jan 24;415(6870):429–433. doi: 10.1038/415429a. [DOI] [PubMed] [Google Scholar]
- Fahim C, Yoon U, Das S, Lyttelton O, Chen J, Arnaoutelis R, et al. Somatosensory-motor bodily representation cortical thinning in Tourette: effects of tic severity, age and gender. Cortex. 2010 Jun;46(6):750–760. doi: 10.1016/j.cortex.2009.06.008. [DOI] [PubMed] [Google Scholar]
- Fotopoulou A, Tsakiris M, Haggard P, Vagopoulou A, Rudd A, Kopelman M. The role of motor intention in motor awareness: an experimental study on anosognosia for hemiplegia. Brain. 2008 Dec;131(Pt 12):3432–3442. doi: 10.1093/brain/awn225. [DOI] [PubMed] [Google Scholar]
- Franck N, Farrer C, Georgieff N, Marie-Cardine M, Dalery J, d'Amato T, et al. Defective recognition of one's own actions in patients with schizophrenia. Am J Psychiatry. 2001 Mar;158(3):454–459. doi: 10.1176/appi.ajp.158.3.454. [DOI] [PubMed] [Google Scholar]
- Frith CD, Blakemore SJ, Wolpert DM. Abnormalities in the awareness and control of action. Philos Trans R Soc Lond B Biol Sci. 2000 Dec 29;355(1404):1771–1788. doi: 10.1098/rstb.2000.0734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garbarini F, Rabuffetti M, Piedimonte A, Pia L, Ferrarin M, Frassinetti F, et al. 'Moving' a paralysed hand: bimanual coupling effect in patients with anosognosia for hemiplegia. Brain. 2012 May;135(Pt 5):1486–1497. doi: 10.1093/brain/aws015. [DOI] [PubMed] [Google Scholar]
- Georgiou N, Bradshaw JL, Phillips JG, Chiu E, Bradshaw JA. Reliance on advance information and movement sequencing in Huntington's disease. Mov Disord. 1995 Jul;10(4):472–481. doi: 10.1002/mds.870100412. [DOI] [PubMed] [Google Scholar]
- Ghosh A, Dutt A. Utilisation behaviour in frontotemporal dementia. J Neurol Neurosurg Psychiatry. 2010 Feb;81(2):154–156. doi: 10.1136/jnnp.2008.160416. [DOI] [PubMed] [Google Scholar]
- Haarmeier T, Bunjes F, Lindner A, Berret E, Thier P. Optimizing visual motion perception during eye movements. Neuron. 2001 Nov 8;32(3):527–535. doi: 10.1016/s0896-6273(01)00486-x. [DOI] [PubMed] [Google Scholar]
- Haggard P, Clark S. Intentional action: conscious experience and neural prediction. Conscious Cogn. 2003 Dec;12(4):695–707. doi: 10.1016/s1053-8100(03)00052-7. [DOI] [PubMed] [Google Scholar]
- Haggard P, Clark S, Kalogeras J. Voluntary action and conscious awareness. Nat Neurosci. 2002 Apr;5(4):382–385. doi: 10.1038/nn827. [DOI] [PubMed] [Google Scholar]
- Haggard P, Martin F, Taylor-Clarke M, Jeannerod M, Franck N. Awareness of action in schizophrenia. Neuroreport. 2003 May 23;14(7):1081–1085. doi: 10.1097/01.wnr.0000073684.00308.c0. [DOI] [PubMed] [Google Scholar]
- Hallett M. Volitional control of movement: the physiology of free will. Clin Neurophysiol. 2007 Jun;118(6):1179–1192. doi: 10.1016/j.clinph.2007.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallett M. Physiology of psychogenic movement disorders. J Clin Neurosci. 2010 Aug;17(8):959–965. doi: 10.1016/j.jocn.2009.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilman KM, Barrett AM, Adair JC. Possible mechanisms of anosognosia: a defect in self-awareness. Philos Trans R Soc Lond B Biol Sci. 1998 Nov 29;353(1377):1903–1909. doi: 10.1098/rstb.1998.0342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson SR, Parkinson A, Kim SY, Schuermann M, Eickhoff SB. On the functional anatomy of the urge-for-action. Cogn Neurosci. 2011 Sep;2(3–4):227–243. doi: 10.1080/17588928.2011.604717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahanshahi M, Hallett M. The Bereitschaftspotential: What does it measure and where does it come from? In: Jahanshahi M, Hallett M, editors. The Bereitschaftspotential. New York: Kluwer Academic/Plenum Publishers; 2003. [Google Scholar]
- Johnson KA, Cunnington R, Iansek R, Bradshaw JL, Georgiou N, Chiu E. Movement-related potentials in Huntington's disease: movement preparation and execution. Exp Brain Res. 2001 Jun;138(4):492–499. doi: 10.1007/s002210100733. [DOI] [PubMed] [Google Scholar]
- Karp BI, Porter S, Toro C, Hallett M. Simple motor tics may be preceded by a premotor potential. J Neurol Neurosurg Psychiatry. 1996 Jul;61(1):103–106. doi: 10.1136/jnnp.61.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoblich G, Stottmeister F, Kircher T. Self-monitoring in patients with schizophrenia. Psychol Med. 2004 Nov;34(8):1561–1569. doi: 10.1017/s0033291704002454. [DOI] [PubMed] [Google Scholar]
- Kranick S, Ekanayake V, Martinez V, Ameli R, Hallett M, Voon V. Psychopathology and psychogenic movement disorders. Mov Disord. 2011 Aug 15;26(10):1844–1850. doi: 10.1002/mds.23830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranick SM, Moore JW, Yusuf N, Martinez VT, LaFever K, Edwards MI, Mehta AR, Collins P, Harrison NA, Haggard P, Hallett M, Voon V. Action-effect binding is decreased in motor conversion disorder: implications for sense of agency. Mov Disord. doi: 10.1002/mds.25408. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumru H, Valls-Sole J, Valldeoriola F, Marti MJ, Sanegre MT, Tolosa E. Transient arrest of psychogenic tremor induced by contralateral ballistic movements. Neurosci Lett. 2004 Nov 11;370(2–3):135–139. doi: 10.1016/j.neulet.2004.08.009. [DOI] [PubMed] [Google Scholar]
- Labate A, Cerasa A, Mula M, Mumoli L, Gioia MC, Aguglia U, et al. Neuroanatomic correlates of psychogenic nonepileptic seizures: a cortical thickness and VBM study. Epilepsia. 2012 Feb;53(2):377–385. doi: 10.1111/j.1528-1167.2011.03347.x. [DOI] [PubMed] [Google Scholar]
- Lerner A, Bagic A, Boudreau EA, Hanakawa T, Pagan F, Mari Z, et al. Neuroimaging of neuronal circuits involved in tic generation in patients with Tourette syndrome. Neurology. 2007 Jun 5;68(23):1979–1987. doi: 10.1212/01.wnl.0000264417.18604.12. [DOI] [PubMed] [Google Scholar]
- Levine DN. Unawareness of visual and sensorimotor defects: a hypothesis. Brain Cogn. 1990 Jul;13(2):233–281. doi: 10.1016/0278-2626(90)90052-p. [DOI] [PubMed] [Google Scholar]
- Lhermitte F. 'Utilization behaviour' and its relation to lesions of the frontal lobes. Brain. 1983 Jun;106(Pt 2):237–255. doi: 10.1093/brain/106.2.237. [DOI] [PubMed] [Google Scholar]
- Libet B, Gleason CA, Wright EW, Pearl DK. Time of conscious intention to act in relation to onset of cerebral activity (readiness-potential). The unconscious initiation of a freely voluntary act. Brain. 1983 Sep;106(Pt 3):623–642. doi: 10.1093/brain/106.3.623. [DOI] [PubMed] [Google Scholar]
- Lindner A, Thier P, Kircher TT, Haarmeier T, Leube DT. Disorders of agency in schizophrenia correlate with an inability to compensate for the sensory consequences of actions. Curr Biol. 2005 Jun 21;15(12):1119–1124. doi: 10.1016/j.cub.2005.05.049. [DOI] [PubMed] [Google Scholar]
- Ludolph AG, Pinkhardt EH, Tebartz van Elst L, Libal G, Ludolph AC, Fegert JM, et al. Are amygdalar volume alterations in children with Tourette syndrome due to ADHD comorbidity? Dev Med Child Neurol. 2008 Jul;50(7):524–529. doi: 10.1111/j.1469-8749.2008.03014.x. [DOI] [PubMed] [Google Scholar]
- Marshall JC, Halligan PW, Fink GR, Wade DT, Frackowiak RS. The functional anatomy of a hysterical paralysis. Cognition. 1997 Jul;64(1):B1–B8. doi: 10.1016/s0010-0277(97)00020-6. [DOI] [PubMed] [Google Scholar]
- McAuley J, Rothwell J. Identification of psychogenic, dystonic, and other organic tremors by a coherence entrainment test. Mov Disord. 2004 Mar;19(3):253–267. doi: 10.1002/mds.10707. [DOI] [PubMed] [Google Scholar]
- Meador KJ, Watson RT, Bowers D, Heilman KM. Hypometria with hemispatial and limb motor neglect. Brain. 1986 Apr;109(Pt 2):293–305. doi: 10.1093/brain/109.2.293. [DOI] [PubMed] [Google Scholar]
- Moore J, Haggard P. Awareness of action: Inference and prediction. Conscious Cogn. 2008 Mar;17(1):136–144. doi: 10.1016/j.concog.2006.12.004. [DOI] [PubMed] [Google Scholar]
- Moore JW, Fletcher PC. Sense of agency in health and disease: a review of cue integration approaches. Conscious Cogn. 2012 Mar;21(1):59–68. doi: 10.1016/j.concog.2011.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moretto G, Schwingenschuh P, Katschnig P, Bhatia KP, Haggard P. Delayed experience of volition in Gilles de la Tourette syndrome. J Neurol Neurosurg Psychiatry. 2011 Dec;82(12):1324–1327. doi: 10.1136/jnnp.2010.221143. [DOI] [PubMed] [Google Scholar]
- Nagaratnam N, Nagaratnam K, Ng K, Diu P. Akinetic mutism following stroke. J Clin Neurosci. 2004 Jan;11(1):25–30. doi: 10.1016/j.jocn.2003.04.002. [DOI] [PubMed] [Google Scholar]
- Obeso JA, Rothwell JC, Marsden CD. Simple tics in Gilles de la Tourette's syndrome are not prefaced by a normal premovement EEG potential. J Neurol Neurosurg Psychiatry. 1981 Aug;44(8):735–738. doi: 10.1136/jnnp.44.8.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papa SM, Artieda J, Obeso JA. Cortical activity preceding self-initiated and externally triggered voluntary movement. Mov Disord. 1991;6(3):217–224. doi: 10.1002/mds.870060305. [DOI] [PubMed] [Google Scholar]
- Peterson BS, Choi HA, Hao X, Amat JA, Zhu H, Whiteman R, et al. Morphologic features of the amygdala and hippocampus in children and adults with Tourette syndrome. Arch Gen Psychiatry. 2007 Nov;64(11):1281–1291. doi: 10.1001/archpsyc.64.11.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips JG, Bradshaw JL, Chiu E, Teasdale N, Iansek R, Bradshaw JA. Bradykinesia and movement precision in Huntington's disease. Neuropsychologia. 1996 Dec;34(12):1241–1245. doi: 10.1016/0028-3932(96)00049-8. [DOI] [PubMed] [Google Scholar]
- Piacentini J, Woods DW, Scahill L, Wilhelm S, Peterson AL, Chang S, et al. Behavior therapy for children with Tourette disorder: a randomized controlled trial. JAMA. 2010 May 19;303(19):1929–1937. doi: 10.1001/jama.2010.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell ER, Kan E, Yoshii J, Thompson PM, Bansal R, Xu D, et al. Thinning of sensorimotor cortices in children with Tourette syndrome. Nat Neurosci. 2008 Jun;11(6):637–639. doi: 10.1038/nn.2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spence SA, Brooks DJ, Hirsch SR, Liddle PF, Meehan J, Grasby PM. A PET study of voluntary movement in schizophrenic patients experiencing passivity phenomena (delusions of alien control) Brain. 1997 Nov;120(Pt 11):1997–2011. doi: 10.1093/brain/120.11.1997. [DOI] [PubMed] [Google Scholar]
- Spence SA, Crimlisk HL, Cope H, Ron MA, Grasby PM. Discrete neurophysiological correlates in prefrontal cortex during hysterical and feigned disorder of movement. Lancet. 2000 Apr 8;355(9211):1243–1244. doi: 10.1016/S0140-6736(00)02096-1. [DOI] [PubMed] [Google Scholar]
- Sperduti M, Delaveau P, Fossati P, Nadel J. Different brain structures related to self- and external-agency attribution: a brief review and meta-analysis. Brain Struct Funct. 2011 Jun;216(2):151–157. doi: 10.1007/s00429-010-0298-1. [DOI] [PubMed] [Google Scholar]
- Stone J, Edwards MJ. How "psychogenic" are psychogenic movement disorders? Mov Disord. 2011 Aug 15;26(10):1787–1788. doi: 10.1002/mds.23882. [DOI] [PubMed] [Google Scholar]
- Synofzik M, Thier P, Leube DT, Schlotterbeck P, Lindner A. Misattributions of agency in schizophrenia are based on imprecise predictions about the sensory consequences of one's actions. Brain. 2010 Jan;133(Pt 1):262–271. doi: 10.1093/brain/awp291. [DOI] [PubMed] [Google Scholar]
- Tanaka Y, Iwasa H, Yoshida M. Diagonistic dyspraxia: case report and movement-related potentials. Neurology. 1990 Apr;40(4):657–661. doi: 10.1212/wnl.40.4.657. [DOI] [PubMed] [Google Scholar]
- Terada K, Ikeda A, Van Ness PC, Nagamine T, Kaji R, Kimura J, et al. Presence of Bereitschaftspotential preceding psychogenic myoclonus: clinical application of jerk-locked back averaging. J Neurol Neurosurg Psychiatry. 1995 Jun;58(6):745–747. doi: 10.1136/jnnp.58.6.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teufel C, Kingdon A, Ingram JN, Wolpert DM, Fletcher PC. Deficits in sensory prediction are related to delusional ideation in healthy individuals. Neuropsychologia. 2010 Dec;48(14):4169–4172. doi: 10.1016/j.neuropsychologia.2010.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomalla G, Siebner HR, Jonas M, Baumer T, Biermann-Ruben K, Hummel F, et al. Structural changes in the somatosensory system correlate with tic severity in Gilles de la Tourette syndrome. Brain. 2009 Mar;132(Pt 3):765–777. doi: 10.1093/brain/awn339. [DOI] [PubMed] [Google Scholar]
- Tiihonen J, Kuikka J, Viinamaki H, Lehtonen J, Partanen J. Altered cerebral blood flow during hysterical paresthesia. Biol Psychiatry. 1995 Jan 15;37(2):134–135. doi: 10.1016/0006-3223(94)00230-Z. [DOI] [PubMed] [Google Scholar]
- Toro C, Torres F. Electrophysiological correlates of a paroxysmal movement disorder. Ann Neurol. 1986 Dec;20(6):731–734. doi: 10.1002/ana.410200614. [DOI] [PubMed] [Google Scholar]
- Voon V, Brezing C, Gallea C, Ameli R, Roelofs K, LaFrance WC, Jr, et al. Emotional stimuli and motor conversion disorder. Brain. 2010 May;133(Pt 5):1526–1536. doi: 10.1093/brain/awq054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voon V, Brezing C, Gallea C, Hallett M. Aberrant supplementary motor complex and limbic activity during motor preparation in motor conversion disorder. Mov Disord. 2011 Nov;26(13):2396–2403. doi: 10.1002/mds.23890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voon V, Gallea C, Hattori N, Bruno M, Ekanayake V, Hallett M. The involuntary nature of conversion disorder. Neurology. 2010 Jan 19;74(3):223–228. doi: 10.1212/WNL.0b013e3181ca00e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss M, Moore J, Hauser M, Gallinat J, Heinz A, Haggard P. Altered awareness of action in schizophrenia: a specific deficit in predicting action consequences. Brain. 2010 Oct;133(10):3104–3112. doi: 10.1093/brain/awq152. [DOI] [PubMed] [Google Scholar]
- Vuilleumier P, Chicherio C, Assal F, Schwartz S, Slosman D, Landis T. Functional neuroanatomical correlates of hysterical sensorimotor loss. Brain. 2001 Jun;124(Pt 6):1077–1090. doi: 10.1093/brain/124.6.1077. [DOI] [PubMed] [Google Scholar]
- Wang Z, Maia TV, Marsh R, Colibazzi T, Gerber A, Peterson BS. The neural circuits that generate tics in Tourette's syndrome. Am J Psychiatry. 2011 Dec;168(12):1326–1337. doi: 10.1176/appi.ajp.2011.09111692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein EA, Kahn RL. Denial of illness: symbolic and physiological aspects. Illinois: Charles C Thomas; 1955. [Google Scholar]
- Worbe Y, Gerardin E, Hartmann A, Valabregue R, Chupin M, Tremblay L, et al. Distinct structural changes underpin clinical phenotypes in patients with Gilles de la Tourette syndrome. Brain. 2010 Dec;133(Pt 12):3649–3660. doi: 10.1093/brain/awq293. [DOI] [PubMed] [Google Scholar]
- Zeuner KE, Shoge RO, Goldstein SR, Dambrosia JM, Hallett M. Accelerometry to distinguish psychogenic from essential or parkinsonian tremor. Neurology. 2003 Aug 26;61(4):548–550. doi: 10.1212/01.wnl.0000076183.34915.cd. [DOI] [PubMed] [Google Scholar]