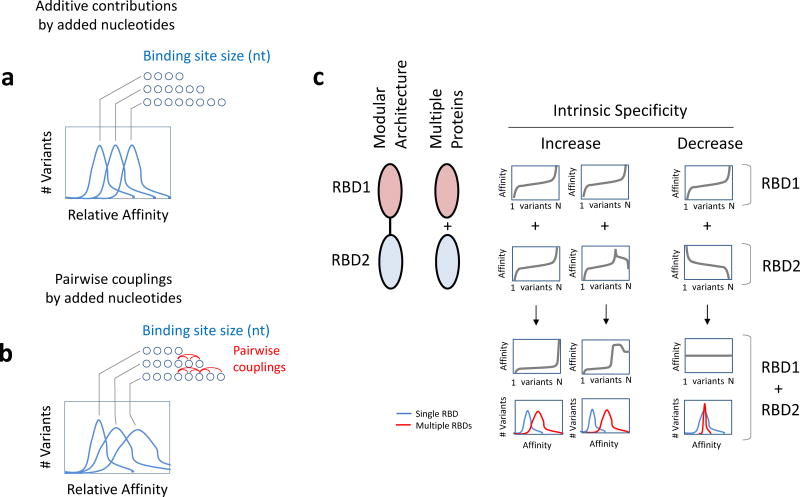
Figure 4. Strategies to increase or decrease intrinsic specificity of RBPs.
(a) Change in binding site size with additive contributions by added nucleotides to binding energy. For a hypothetical RBP, additional nucleotides in a binding site would shift the affinity distribution towards higher affinities, but would not necessarily broaden the affinity distribution and thus not increase inherent specificity. (b) Change in binding site size with contributions of pairwise energetic couplings by added nucleotides. For a hypothetical RBP, hypothetical pairwise couplings by additional nucleotides in the binding site could strongly favor a small number of nucleotide combinations, thereby broaden the affinity distribution and thus greatly increase inherent specificity. (c) Increase or decrease in intrinsic specificity through multiple RBDs. Multiple RBDs (RBD1 and RBD2) can be part of the same protein or of separate proteins (left). The panels on the right show ranked affinity distributions (according to the same sequences for both RBDs) for each RDB. The panels in row three show the ranked affinity distribution upon combination of both RBDs, and the corresponding histogram of this ranked affinity distribution, color coded as indicated. Inherent protein specificity can be increased by additive specificities of the RBDs or decreased by compensatory specificities. Intrinsic specificities for individual RBDs can vary. Note, however, that binding preferences of individual RBDs do not need to be strictly additive, but can be synergistic, either through interactions between the RBDs or through cooperative binding of multiple several proteins.