Abstract
Background
Lixisenatide is a once‐daily, prandial, short‐acting glucagon‐like peptide‐1 receptor agonist. Its main antidiabetic effect is to delay gastric emptying to control postprandial plasma glucose excursions. The dose–response relationship of the integrated insulinotropic and gastrostatic response to lixisenatide in healthy volunteers after a standardized liquid meal was investigated.
Methods
Twenty healthy subjects received acetaminophen 1000 mg with a standardized liquid meal 60 min after a single subcutaneous injection of placebo or lixisenatide 2.5, 5, 10 or 20 µg in randomized order separated by a 2‐ to 7‐day washout. Acetaminophen pharmacokinetics served as a surrogate to assess rate of gastric emptying. Postprandial plasma glucose, insulin, C‐peptide and glucagon were assessed for 5 h after the meal test, and lixisenatide pharmacokinetics were determined for 6 h.
Results
After lixisenatide administration and prior to the standardized meal, insulin and C‐peptide transiently increased, while fasting plasma glucose decreased in a dose‐dependent manner. After the meal, postprandial plasma glucose, insulin and C‐peptide were dose proportionally reduced with lixisenatide versus placebo for up to 6 h. Compared with placebo, glucagon levels were transiently lower after any lixisenatide dose, with more sustained reductions after the meal and no apparent dose‐related trends. Acetaminophen absorption was significantly reduced and delayed compared with placebo for lixisenatide doses ≥5 µg and demonstrated dose‐dependent slowing of gastric emptying. Lixisenatide displayed near dose‐proportional exposure, with gastrointestinal events increasing with dose.
Conclusions
Lixisenatide reduced fasting plasma glucose via stimulation of glucose‐dependent insulin release and controlled postprandial plasma glucose by delaying gastric emptying, demonstrating it to be a valuable option for overall glycaemic control. Copyright © 2015 John Wiley & Sons, Ltd.
Keywords: healthy subjects, lixisenatide, gastric emptying, glycaemic control, pharmacokinetics, type 2 diabetes mellitus
Introduction
Both postprandial and fasting plasma glucose (PPG and FPG respectively) contribute to overall glycated haemoglobin (HbA1c) levels, with the relative importance of PPG and FPG depending on several factors, including current treatment regimens and actual HbA1c level 1, 2, 3. In patients with type 2 diabetes mellitus (T2DM) receiving basal insulin, with resulting well‐controlled FPG, the contribution of PPG to overall HbA1c levels seems to be especially prominent 3. A major determinant of PPG control is the rate of gastric emptying, accounting for up to 35% of the variance in response to a meal or glucose challenge 4. Gastric emptying is accelerated in hypoglycaemia and slowed in hyperglycaemia, in both healthy subjects and those with T2DM 5, 6, 7, 8. Furthermore, patients with diabetes with gastroparesis have blunted PPG excursions and reduced insulin use 6, 9, and pharmacologically induced delay of gastric emptying is being pursued as a method of improving glycaemic control.
Glucagon‐like peptide‐1 (GLP‐1) is a naturally occurring, gut‐derived incretin hormone 10, 11. It is released postprandially and involved in the stimulation of insulin secretion and the suppression of glucagon release in the pancreas, and delay of gastric emptying in the stomach. GLP‐1 receptor agonists (GLP‐1 RAs) mimic the activity of GLP‐1 while also having a prolonged half‐life and more long‐lasting activity compared with endogenous GLP‐1 12. Delay of gastric emptying, over and above stimulation of glucose‐dependent insulin release, is thought to be the predominant determinant of PPG control with the once‐daily, short‐acting prandial GLP‐1 RA lixisenatide (Lyxumia®, Sanofi, Paris, France) 13 and has been shown to be an important mechanism for control of PPG with the prandial GLP‐1 RA exenatide twice‐daily (BID) 14.
A recent study aiming to elucidate the insulinotropic effect of lixisenatide assessed glucose disposal after an intravenous glucose challenge and revealed that lixisenatide can resensitize glucose‐dependent insulin release in patients with T2DM, particularly in individuals with early‐stage disease and modestly elevated HbA1c levels. In the presence of lixisenatide, glucose disposal was returned to almost normal intensities, without impairing counter‐regulation to low glucose by glucagon 15.
The primary objective of this study was to investigate the integrated insulinotropic and gastrostatic response of lixisenatide on PPG, and corresponding insulin and glucagon release in healthy volunteers after an oral glucose challenge (standardized meal). A further aim was to establish the pharmacokinetic and pharmacodynamic dose–response relationship between lixisenatide and gastric emptying.
Materials and methods
Study design
This was a Phase I, single‐centre, randomized, open‐label, placebo‐controlled, crossover (5‐sequence, 5‐period, 5‐treatment) study conducted at PAREXEL International GmbH, Berlin, Germany, from January to March 2013. The study protocol was submitted to an Independent Ethics Committee for review and written approval, and the study was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice Guidelines. All patients provided written informed consent prior to any procedure that was related to the study.
Study participants
Subjects were volunteers aged 18–45 years with a body mass index of 18–28 kg m−2, inclusive. All subjects were certified healthy by a comprehensive clinical assessment.
Treatment
Each of the five treatment periods lasted 1 day, followed by a 2‐ to 7‐day washout period before the next treatment period. An end‐of‐study visit occurred 2–7 days after the last treatment period. The overall duration of observation was approximately 6 weeks for each subject. At 3–6 months after the last treatment period, subjects had the option of attending a post‐study visit if they had converted to being anti‐lixisenatide antibody‐positive at the end‐of‐study visit.
After an overnight fast of at least 10 h, single doses of lixisenatide 2.5, 5, 10 or 20 µg or matched placebo were administered to each subject into the abdominal subcutis during one of the five distinct treatment periods. At 60‐min post‐lixisenatide or post‐placebo administration, a standardized liquid meal (400 mL of Ensure Plus Next Generation Vanilla [600 kcal]) was administered and followed within 10–15 min by oral acetaminophen 1000 mg. A 1000‐mg dose of acetaminophen is a commonly administered therapeutic dose in adults and was expected to produce adequate concentrations for pharmacodynamic analysis. Acetaminophen absorption has been shown to be reliably dependent on the rate of gastric emptying 16 and, hence, is commonly used as a surrogate for gastric emptying.
Blood specimens for determination of lixisenatide, acetaminophen and glucose, insulin, C‐peptide and glucagon were taken at predefined times from 90 min before to 300 min after meal intake.
Assessments
The primary endpoint was the area under the PPG curve from 0 to 1 h after the meal challenge (PPG–AUC0–1 h). Secondary pharmacodynamic endpoints included area under the serum insulin concentration curve from 0 to 1 h after meal intake (INS–AUC0–1 h), maximum plasma glucose and maximum serum insulin concentrations (PPG–Cmax and INS–Cmax respectively) and time of PPG–Cmax and INS–Cmax (PPG–t max and INS–t max respectively). Mean serum glucagon and plasma C‐peptide concentrations were also measured. Pharmacokinetic parameters included the area under the lixisenatide plasma concentration time curve to the last observed time (LIXI–AUClast) and extrapolated to infinity (LIXI–AUC), fractional areas under the plasma acetaminophen curve from 0 to 5 h after meal challenge (ACT–AUC0–x h; where x is 1, 2, 3, 4 or 5 h), as well as maximum plasma lixisenatide and acetaminophen concentrations (LIXI–Cmax and ACT–Cmax), and time of LIXI–Cmax and ACT–Cmax (LIXI–t max and ACT–t max). Safety endpoints included adverse events (AEs) and serious AEs reported by subject/observed by investigator and bedside blood glucose tests.
Statistical analysis
Pharmacodynamics
PPG–AUC0–1 h, PPG–Cmax, INS–AUC0–1 h and INS–Cmax were analysed using an analysis of covariance model with treatment, sequence and period as fixed effects, subjects within sequence as a random effect and the corresponding baseline (T –0.5 h) levels as a covariate. The least squares mean differences between treatment groups and 90% confidence intervals (CIs) were calculated within the model framework. For PPG–t max and INS–t max, descriptive statistics were provided.
Pharmacokinetics
Pharmacokinetic parameters of acetaminophen and lixisenatide were summarized by treatment using descriptive statistics. Log‐transformed acetaminophen and lixisenatide pharmacokinetic parameters were compared among the five acetaminophen (i.e. four lixisenatide arms and one placebo arm) or four lixisenatide treatments using a linear mixed effects model, with fixed terms of treatment, sequence and period, with an unstructured 5 × 5 (4 × 4 for lixisenatide) matrix for treatment‐specific variances and covariances for subject within sequence. For all parameters, estimates and 90% CIs for the ratio of treatment means were computed for the differences between treatment means within the linear mixed effects model framework, and then converted to ratios by the antilog transformation.
Results
Population characteristics
Twenty healthy volunteers were randomized and commenced treatment. A summary of demographics and subject characteristics is shown in Table 1. A total of 19 subjects completed all five treatment periods. One subject withdrew consent because of personal reasons after completion of treatment period 3. At the time of leaving the study, the subject had failed to complete the placebo and lixisenatide 20‐µg treatment periods.
Table 1.
Demographics and subject characteristics at baseline, safety population
| All subjects (N = 20) | |
|---|---|
| Age (years) | |
| Mean (SD) | 31.0 (7.3) |
| Min:Max | 18:44 |
| Sex [n (%)] | |
| Male | 10 (50.0) |
| Female | 10 (50.0) |
| Race [n (%)] | |
| Caucasian/White | 19 (95.0) |
| Asian/Oriental | 1 (5.0) |
| Height (cm) | |
| Mean (SD) | 174.2 (11.5) |
| Min:Max | 155:192 |
| Weight (kg) | |
| Mean (SD) | 69.7 (14.6) |
| Min:Max | 51.7:99.9 |
| BMI (kg m−2) | |
| Mean (SD) | 22.8 (2.7) |
| Min:Max | 18.3:27.1 |
BMI, body mass index; SD, standard deviation.
Pharmacodynamics
Prior to the standardized meal
Before the standardized meal, FPG generally decreased in a dose‐dependent manner after the lixisenatide injection compared with placebo (Figure 1a). Corresponding with the reduction in FPG, small and transient increases in insulin (Figure 1b) and C‐peptide (Figure 1c) concentration were observed. Additionally, there appeared to be a slight decrease in glucagon concentrations from 1 h before the meal (Figure 1d).
Figure 1.
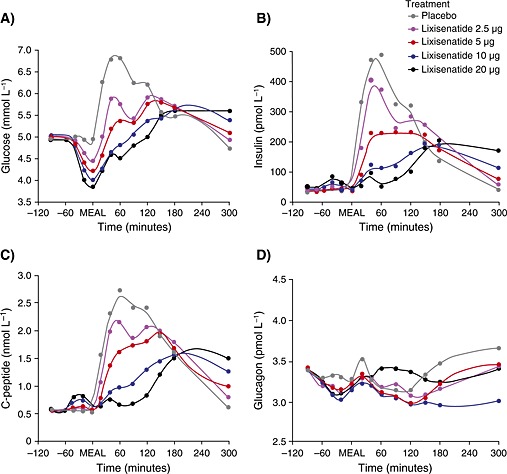
Mean (A) plasma glucose (mmol L−1), (B) insulin (pmol L−1), (C) C‐peptide (nmol L−1) and (D) glucagon (pmol L−1) over time from −120 to 300 min. ‘MEAL’ marks the meal challenge time point (60 min after lixisenatide administration)
After the standardized meal
For the primary endpoint (PPG–AUC0–1 h) and secondary endpoints (INS–AUC0–1 h, PPG–Cmax and INS–Cmax), a general dose‐dependent treatment effect was demonstrated for lixisenatide 2.5 to 20 µg (Table 2 [PPG] and Table 3 [INS]), with no substantial difference between lixisenatide 10 and 20 µg. Compared with placebo, a dose‐dependent reduction in PPG was demonstrated with lixisenatide 2.5 to 20 µg (Figure 1a), again without a substantial difference between lixisenatide 10 and 20 µg. Corresponding to the reduction in PPG, insulin (Figure 1b) and also C‐peptide concentration (Figure 1c) were reduced compared with placebo in a dose‐dependent manner up to 300 min after the meal. Lixisenatide 20 µg produced a more sustained delay in insulin secretion than lixisenatide 10 µg. While glucagon levels were transiently lower after any lixisenatide dose with more sustained reductions after the meal, in contrast with insulin, there were no apparent dose‐related trends in plasma glucagon concentrations compared with placebo (Figure 1d).
Table 2.
Pharmacodynamic outcomes for primary endpoint PPG–AUC0–1 h and secondary endpoint PPG–Cmax
| Parameter | Lixisenatide treatment group | Effect estimate vs placebo | 90% confidence intervals | p‐value |
|---|---|---|---|---|
| PPG–AUC0–1 h (mmol min L−1) | 2.5 µg | −61.2 | −75.2, −47.3 | <0.001 |
| 5 µg | −92.3 | −106.2, −78.4 | <0.001 | |
| 10 µg | −118.4 | −132.4, −104.4 | <0.001 | |
| 20 µg | −121.1 | −135.2, −107.1 | <0.001 | |
| PPG–Cmax (mmol L−1) | 2.5 µg | −0.8 | −1.1, −0.5 | <0.001 |
| 5 µg | −1.0 | −1.3, −0.7 | <0.001 | |
| 10 µg | −1.5 | −1.8, −1.2 | <0.001 | |
| 20 µg | −1.2 | −1.6, −0.9 | <0.001 | |
| Parameter | Lixisenatide treatment group | Effect estimate between doses | 90% confidence intervals | p‐value |
| PPG–AUC0–1 h (mmol min L−1) | 5 µg vs 2.5 µg | −31.1 | −44.7, −17.4 | <0.001 |
| 10 µg vs 2.5 µg | −57.2 | −70.9, −43.4 | <0.001 | |
| 20 µg vs 2.5 µg | −59.9 | −73.8, −46.0 | <0.001 | |
| 10 µg vs 5 µg | −26.1 | −39.8, −12.4 | 0.002 | |
| 20 µg vs 5 µg | −28.9 | −42.8, −14.9 | <0.001 | |
| 20 µg vs 10 µg | −2.8 | −16.7, 11.2 | NS | |
| PPG–Cmax (mmol L−1) | 5 µg vs 2.5 µg | −0.3 | −0.6, 0.1 | NS |
| 10 µg vs 2.5 µg | −0.7 | −1.0, −0.4 | <0.001 | |
| 20 µg vs 2.5 µg | −0.5 | −0.8, −0.2 | 0.015 | |
| 10 µg vs 5 µg | −0.5 | −0.8, −0.2 | 0.015 | |
| 20 µg vs 5 µg | −0.2 | −0.6, 0.1 | NS | |
| 20 µg vs 10 µg | 0.2 | −0.1, 0.6 | NS |
AUC, area under the curve; Cmax, maximum concentration; NS, non‐significant p‐value; PPG, postprandial plasma glucose.
Data are point estimates of treatment group differences with 90% confidence intervals for lixisenatide treatment groups (2.5, 5, 10 and 20 µg) versus placebo or between lixisenatide doses.
Table 3.
Pharmacodynamic outcomes for secondary endpoint INS–AUC0–1 h and INS–Cmax
| Parameter | Lixisenatide treatment group | Effect estimate vs placebo | 90% confidence intervals | p‐value |
|---|---|---|---|---|
| INS–AUC0–1 h (pmol min L−1) | 2.5 µg | −8648 | −12,713, −4584 | <0.001 |
| 5 µg | −16,762 | −20,848, −12,676 | <0.001 | |
| 10 µg | −21,896 | −25,936, −17,856 | <0.001 | |
| 20 µg | −24,848 | −29,078, −20,618 | <0.001 | |
|
INS–Cmax
(pmol L−1) |
2.5 µg | −101 | −216, 15 | NS |
| 5 µg | −277 | −391, −164 | <0.001 | |
| 10 µg | −428 | −543, −314 | <0.001 | |
| 20 µg | −388 | −509, −268 | <0.001 | |
| Parameter | Lixisenatide treatment group | Effect estimate between doses | 90% confidence intervals | p‐value |
| INS–AUC0–1 h (pmol min L−1) | 5 µg vs 2.5 µg | −8114 | −12,173, −4055 | 0.001 |
| 10 µg vs 2.5 µg | −13,248 | −17,214, −9281 | <0.001 | |
| 20 µg vs 2.5 µg | −16,200 | −20,262, −12,137 | <0.001 | |
| 10 µg vs 5 µg | −5134 | −9170, −1098 | 0.038 | |
| 20 µg vs 5 µg | −8086 | −12,322, −3850 | 0.002 | |
| 20 µg vs 10 µg | −2952 | −7049, 1145 | NS | |
| INS–Cmax (pmol L−1) | 5 µg vs 2.5 µg | −177 | −289, −64 | 0.011 |
| 10 µg vs 2.5 µg | −328 | −440, −216 | <0.001 | |
| 20 µg vs 2.5 µg | −288 | −403, −173 | <0.001 | |
| 10 µg vs 5 µg | −151 | −263, −40 | 0.027 | |
| 20 µg vs 5 µg | −111 | −229, 6 | NS | |
| 20 µg vs 10 µg | 40 | −76, 156 | NS |
AUC, area under the curve; Cmax, maximum concentration; INS, insulin; NS, non‐significant p‐value.
Data are point estimates of treatment group differences with 90% confidence intervals for lixisenatide treatment groups (2.5, 5, 10 and 20 µg) versus placebo or between lixisenatide doses.
Pharmacokinetics
Lixisenatide
Lixisenatide exposure increased in a slightly less than dose‐proportional manner (Figure 2a). LIXI–AUC and LIXI–Cmax increased 0.85‐fold (90% CI 0.78, 0.91) and 0.77‐fold (90% CI 0.72, 0.82) respectively (data not shown). For lixisenatide 2.5 to 10 µg, LIXI–t max was reached at a median of 1.5 h after meal administration versus a median of 2.0 h with lixisenatide 20 µg (data not shown).
Figure 2.
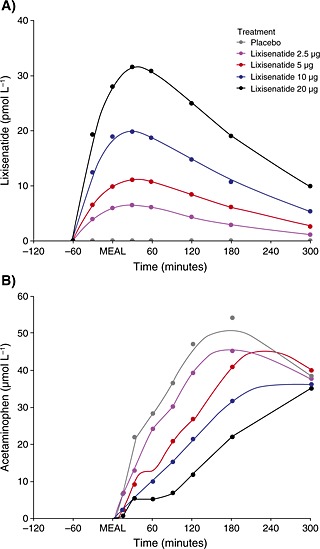
Mean (A) lixisenatide plasma time profiles from 0 to 360 min and (B) acetaminophen plasma time profiles (as a surrogate measure for gastric emptying) for different doses of lixisenatide over time from −120 to 300 min. ‘MEAL’ marks the meal challenge time point (60 min after lixisenatide administration)
Acetaminophen
Fractional ACT–AUC0–1 h, ACT–AUClast and ACT–Cmax decreased with lixisenatide dose, while ACT–t max increased from 3 to 5 h after the meal (Table 4); for AUC0–1 h and Cmax, these differences were significant (p < 0.05) compared with placebo for lixisenatide doses of 5 µg or more. Both Figure 2b, which shows the mean plasma acetaminophen concentrations versus the different doses of lixisenatide over time, and Figure 3a, which displays the ratios of cumulative hourly acetaminophen exposure to placebo, demonstrate the delaying effect of lixisenatide on gastric emptying. Figure 3a shows that lixisenatide doses >2.5 µg given 1 h prior to the standardized liquid meal significantly blunted gastric emptying by, on average, more than 50% up to a maximum of >80%. For lixisenatide 5 and 10 µg, the rate of gastric emptying began to gradually recover 2 h after the meal, while with lixisenatide 20 µg, the initial inhibition of gastric emptying was prolonged for an additional hour. Although acetaminophen concentrations were comparable at 3‐h post‐meal among the lower doses of lixisenatide, cumulative exposure of acetaminophen and, hence, glucose absorption continued to be incomplete compared with placebo beyond the 6‐h observation period with all doses, with the overall recovery possibly taking more than 12 h (data not shown). Figure 3b displays the inverse relationship between lixisenatide exposure and acetaminophen absorption. The lixisenatide exposure required to achieve a reduction of more than 50% in acetaminophen absorption 2 h after the liquid meal was estimated to be on average 244 (interquartile range of 151–357) ng h mL−1. This was achieved in all subjects treated with lixisenatide dose ≥10 µg.
Table 4.
Pharmacokinetic data for acetaminophen by lixisenatide dose administered
| Placebo | Lixisenatide 2.5 µg | Lixisenatide 5 µg | Lixisenatide 10 µg | Lixisenatide 20 µg | |
|---|---|---|---|---|---|
| N | 19 | 20 | 20 | 18 | 16 |
| ACT–Cmax (µg mL−1) | 9.9 (5.2) | 8.7 (2.5) | 7.4 (2.3) | 5.9 (2.0) | 5.9 (2.4) |
| ACT–t max (h), median (min–max) | 3.0 (0.5–5.1) | 3.0 (0.3–5.0) | 3.0 (0.5–5.1) | 5.0 (0.5–5.2) | 5.0 (0.5–5.1) |
| ACT–AUC0–1 h (µg h mL−1) | 2.6 (3.2) | 1.9 (2.2) | 1.1 (1.8) | 0.8 (1.2) | 0.5 (1.2) |
| ACT–AUClast (µg h mL−1) | 29.9 (9.0) | 25.6 (6.6) | 21.6 (6.2) | 17.4 (5.1) | 12.9 (6.0) |
ACT, acetaminophen; AUC, area under the curve; Cmax, maximum concentration; t max, time to maximum concentration.
Data are mean (standard deviation) unless stated otherwise, summarizing the dose‐dependent behaviour of lixisenatide in plasma.
Figure 3.
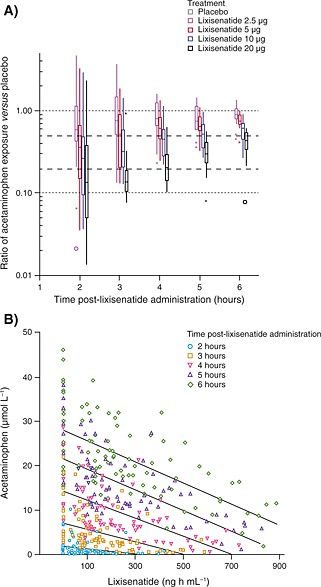
(A) Ratios of cumulative hourly acetaminophen exposure* and (B) the inverse relationship between lixisenatide exposure and acetaminophen absorption, for different doses of lixisenatide over time *Placebo at each time point can be seen at a ratio of 1.0. Values that fall within 1.5–3 times the interquartile range are plotted with asterisks. Far‐outside values that are >3.0 times the interquartile range are plotted with open circles
Safety endpoints (data not shown)
No serious AEs were reported, and no subjects discontinued the study because of treatment‐emergent AEs. The most frequent treatment‐emergent AEs were nausea and vomiting, which increased in a dose‐dependent manner. Blood glucose levels below the lower limit of normal (3.5 mmol L−1) were detected in 3/20, 5/20 and 10/19 subjects administered lixisenatide 5, 10 and 20 µg respectively. One subject had mild hypoglycaemic symptoms beginning approximately 50 min after the administration of lixisenatide 20 µg, with blood glucose levels decreasing from 4.8 (predose) to 3.0 mmol L−1 at 60 min post‐dose. Within 30 min of the standardized meal (given at the normal time of 1 h post‐lixisenatide injection), the subject's blood glucose had increased to 4.1 mmol L−1, and to 4.3 mmol L−1 after an additional 30 min, when all hypoglycaemic symptoms had resolved.
Discussion
In this study of healthy volunteers, lixisenatide dose‐dependently decreased FPG, transiently elevated fasting insulin concentrations and effectively reduced PPG excursions after a standardized meal challenge compared with placebo. Reductions in PPG excursions were associated with slowed gastric emptying (indicated by reduced acetaminophen exposure) and reduced postprandial insulin secretion compared with placebo. This suggests that delayed gastric emptying, rather than insulinotropic effects, is the main driver of PPG reduction with lixisenatide.
The exposure–effect relationships (pharmacokinetics/pharmacodynamics) for the different parameters in this study showed that lixisenatide at a dose as low as 2.5 µg had minor effects on PPG with little impact on gastric emptying, while lixisenatide 5 µg demonstrated a significant reduction in PPG and delay of gastric emptying. The maximum effect on PPG was reached with lixisenatide 10 µg, while lixisenatide 20 µg demonstrated the greatest delays in gastric emptying. The absorption of acetaminophen, and hence the recovery of gastric emptying, was incomplete at the end of the 3‐h observation period following administration of all doses of lixisenatide. In contrast to insulin, glucagon was transiently lower after any lixisenatide dose; this reduction was more sustained after the meal. Although no difference was observed between the different lixisenatide doses in terms of changes in glucagon levels, this effect is in line with the suppression of glucagon release through elevation of insulin secretion with lixisenatide, a mechanism visible in the fasting state, and with enhanced glucose‐dependent stimulation of insulin release in the fed state.
The validity of using acetaminophen absorption to measure the rate of gastric emptying compared with scintigraphy, considered to be the gold standard for assessing gastric emptying, has been evaluated in a systematic literature review 17. Eight of 13 identified studies showed a good correlation between gastric emptying assessed by acetaminophen absorption and scintigraphy, and the general conclusion of the review was that the acetaminophen absorption technique is a valuable tool for clinical use and research purposes. Acetaminophen absorption has been used as a proxy for gastric emptying in a number of earlier studies of GLP‐1 RAs that clearly differentiated the effects of long‐acting and prandial, short‐acting agents. In these studies, exenatide BID substantially slowed gastric emptying in healthy volunteers and patients with T2DM, while liraglutide and placebo demonstrated equivalent effects on acetaminophen exposure 18, 19, 20. Moreover, these significant reductions in gastric emptying with exenatide BID have been confirmed using scintigraphy 14. Sustained plasma concentrations of long‐acting GLP‐1 RAs, such as liraglutide, result in pronounced reductions in FPG but also lead to tachyphylaxis of the delay in gastric emptying, limiting their effect on PPG 21, 22, 23, 24, 25. Indeed, in a head‐to‐head study, lixisenatide has demonstrated significant delays in gastric emptying versus liraglutide 26 and these changes in gastric emptying correlated with significant reductions in PPG with lixisenatide 13. Of interest, a randomized, crossover study comparing exenatide BID with the dipeptidyl peptidase‐4 inhibitor sitagliptin 27 reported that the short‐acting GLP‐1 RA resulted in significantly greater reductions in 2‐h PPG and significantly greater delays in gastric emptying compared with sitagliptin. These findings differentiate prandial, short‐acting GLP‐1 RAs from this alternative incretin‐based approach in terms of achieving optimal postprandial glycaemic control.
While this Phase I study was conducted in healthy volunteers, the Phase III GetGoal trial programme has demonstrated that lixisenatide treatment can bring about a placebo‐subtracted change from baseline in 2‐h PPG of between −3.2 and −7.8 mmol L−1 and in HbA1c of between −0.32 and −0.88%, with an accompanying beneficial effect on body weight 28, 29, 30, 31, 32, 33, 34, 35, 36. Importantly, elevated PPG excursions (more than FPG) are a strong predictor of cardiovascular disease and all‐cause mortality 37. Whether better control of PPG with short‐acting GLP‐1 RAs results in improved cardiovascular outcomes is, as yet, unknown; however, the ongoing Evaluation of Lixisenatide in Acute coronary syndrome study should be informative in this matter. Evaluation of Lixisenatide in Acute coronary syndrome is an event‐driven cardiovascular outcome study in 6000 patients with high cardiovascular risk (defined as patients who recently experienced an acute coronary event). Complete results are scheduled to be available in 2015.
In summary, lixisenatide treatment resulted in dose‐dependent reduction of FPG by stimulation of glucose‐sensitive insulin release and effective reduction of PPG after a meal by delaying gastric emptying, demonstrating it to be a valuable option for overall glycaemic control.
Conflicts of interest
Reinhard Becker, Jens Stechl and Franck Pellissier are employees of Sanofi. Axel Steinstraesser is a consultant and shareholder at Sanofi. Georg Golor is an employee of PAREXEL International GmbH.
Acknowledgements
This Phase 1 study was sponsored by Sanofi. Editorial assistance was provided by Christina Untersperger, PhD, of Caudex Medical and was funded by Sanofi.
Reinhard Becker initiated the investigation, reviewed and interpreted the data, wrote, reviewed and edited the manuscript and approved the final manuscript for submission. Reinhard Becker is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Jens Stechl jointly supervised the investigation, reviewed and edited the manuscript and approved the final manuscript for submission. Axel Steinstraesser and Franck Pellissier reviewed and interpreted the data, provided input into the development of the manuscript and approved the final manuscript for submission. Georg Golor conducted the study and reviewed, edited and approved the final manuscript for submission.
Becker, R. H. A. , Stechl, J. , Steinstraesser, A. , Golor, G. , and Pellissier, F. (2015) Lixisenatide reduces postprandial hyperglycaemia via gastrostatic and insulinotropic effects. Diabetes Metab Res Rev, 31: 610–618. doi: 10.1002/dmrr.2647.
The copyright line for this article was changed on 9 September 2015 after original online publication.
References
- 1. Bonora E, Calcaterra F, Lombardi S, et al. Plasma glucose levels throughout the day and HbA(1c) interrelationships in type 2 diabetes: implications for treatment and monitoring of metabolic control. Diabetes Care 2001; 24(12): 2023–2029. [DOI] [PubMed] [Google Scholar]
- 2. Monnier L, Lapinski H, Colette C. Contributions of fasting and postprandial plasma glucose increments to the overall diurnal hyperglycemia of type 2 diabetic patients: variations with increasing levels of HbA(1c). Diabetes Care 2003; 26(3): 881–885. [DOI] [PubMed] [Google Scholar]
- 3. Riddle M, Umpierrez G, DiGenio A, Zhou R, Rosenstock J. Contributions of basal and postprandial hyperglycemia over a wide range of A1C levels before and after treatment intensification in type 2 diabetes. Diabetes Care 2011; 34(12): 2508–2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Marathe CS, Rayner CK, Jones KL, Horowitz M. Relationships between gastric emptying, postprandial glycemia, and incretin hormones. Diabetes Care 2013; 36(5): 1396–1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Deane AM, Nguyen NQ, Stevens JE, et al. Endogenous glucagon‐like peptide‐1 slows gastric emptying in healthy subjects, attenuating postprandial glycemia. J Clin Endocrinol Metab 2010; 95(1): 215–221. [DOI] [PubMed] [Google Scholar]
- 6. Gonlachanvit S, Hsu CW, Boden GH, et al. Effect of altering gastric emptying on postprandial plasma glucose concentrations following a physiologic meal in type‐II diabetic patients. Dig Dis Sci 2003; 48(3): 488–497. [DOI] [PubMed] [Google Scholar]
- 7. Jones KL, Horowitz M, Carney BI, Wishart JM, Guha S, Green L. Gastric emptying in early noninsulin‐dependent diabetes mellitus. J Nucl Med 1996; 37(10): 1643–1648. [PubMed] [Google Scholar]
- 8. Woerle HJ, Albrecht M, Linke R, et al. Importance of changes in gastric emptying for postprandial plasma glucose fluxes in healthy humans. Am J Physiol Endocrinol Metab 2008; 294(1): E103–E109. [DOI] [PubMed] [Google Scholar]
- 9. Ishii M, Nakamura T, Kasai F, Onuma T, Baba T, Takebe K. Altered postprandial insulin requirement in IDDM patients with gastroparesis. Diabetes Care 1994; 17(8): 901–903. [DOI] [PubMed] [Google Scholar]
- 10. Gutniak M, Orskov C, Holst JJ, Ahren B, Efendic S. Antidiabetogenic effect of glucagon‐like peptide‐1 (7‐36)amide in normal subjects and patients with diabetes mellitus. N Engl J Med 1992; 326(20): 1316–1322. [DOI] [PubMed] [Google Scholar]
- 11. Nauck MA, Kleine N, Orskov C, Holst JJ, Willms B, Creutzfeldt W. Normalization of fasting hyperglycaemia by exogenous glucagon‐like peptide 1 (7‐36 amide) in type 2 (non‐insulin‐dependent) diabetic patients. Diabetologia 1993; 36(8): 741–744. [DOI] [PubMed] [Google Scholar]
- 12. Gallwitz B, Ropeter T, Morys‐Wortmann C, Mentlein R, Siegel EG, Schmidt WE. GLP‐1‐analogues resistant to degradation by dipeptidyl‐peptidase IV in vitro. Regul Pept 2000; 86(1‐3): 103–111. [DOI] [PubMed] [Google Scholar]
- 13. Menge BA, Kapitza C, Hincelin‐Mery A, et al Impact of baseline gastric emptying on effects of lixisenatide and liraglutide in type 2 diabetes mellitus as add‐on to insulin glargine. Presented at the European Association for the Study of Diabetes, 50th Annual Meeting, Vienna, Austria, 15‐19 September 2014, OP75.
- 14. Linnebjerg H, Park S, Kothare PA, et al. Effect of exenatide on gastric emptying and relationship to postprandial glycemia in type 2 diabetes. Regul Pept 2008; 151(1‐3): 123–129. [DOI] [PubMed] [Google Scholar]
- 15. Becker RHA, Stechl J, Msihid J, Kapitza C. Lixisenatide resensitizes the insulin secretory response to intravenous glucose challenge in people with type 2 diabetes ‐ a study in both people with type 2 diabetes and healthy subjects. Diabetes Obes Metab 2014; 16(9): 793–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Heading RC, Nimmo J, Prescott LF, Tothill P. The dependence of paracetamol absorption on the rate of gastric emptying. Br J Pharmacol 1973; 47(2): 415–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Willems M, Quartero AO, Numans ME. How useful is paracetamol absorption as a marker of gastric emptying? a systematic literature study. Dig Dis Sci 2001; 46(10): 2256–2262. [DOI] [PubMed] [Google Scholar]
- 18. Kolterman OG, Kim DD, Shen L, et al. Pharmacokinetics, pharmacodynamics, and safety of exenatide in patients with type 2 diabetes mellitus. Am J Health Syst Pharm 2005; 62(2): 173–181. [DOI] [PubMed] [Google Scholar]
- 19. Blase E, Taylor K, Gao HY, Wintle M, Fineman M. Pharmacokinetics of an oral drug (acetaminophen) administered at various times in relation to subcutaneous injection of exenatide (exendin‐4) in healthy subjects. J Clin Pharmacol 2005; 45(5): 570–577. [DOI] [PubMed] [Google Scholar]
- 20. Kapitza C, Zdravkovic M, Hindsberger C, Flint A. The effect of the once‐daily human glucagon‐like peptide 1 analog liraglutide on the pharmacokinetics of acetaminophen. Adv Ther 2011; 28(8): 650–660. [DOI] [PubMed] [Google Scholar]
- 21. Meier JJ. GLP‐1 receptor agonists for individualized treatment of type 2 diabetes mellitus. Nat Rev Endocrinol 2012; 8(12): 728–742. [DOI] [PubMed] [Google Scholar]
- 22. Doyle ME, Egan JM. Mechanisms of action of glucagon‐like peptide 1 in the pancreas. Pharmacol Ther 2007; 113(3): 546–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Drucker DJ, Nauck MA. The incretin system: glucagon‐like peptide‐1 receptor agonists and dipeptidyl peptidase‐4 inhibitors in type 2 diabetes. Lancet 2006; 368(9548): 1696–1705. [DOI] [PubMed] [Google Scholar]
- 24. Holst JJ. The physiology of glucagon‐like peptide 1. Physiol Rev 2007; 87(4): 1409–1439. [DOI] [PubMed] [Google Scholar]
- 25. Nauck MA, Kemmeries G, Holst JJ, Meier JJ. Rapid tachyphylaxis of the glucagon‐like peptide 1‐induced deceleration of gastric emptying in humans. Diabetes 2011; 60(5): 1561–1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Meier JJ, Rosenstock J, Hincelin‐Mery A, et al Effect of lixisenatide vs liraglutide on glycemic control, gastric emptying and safety parameters in optimized insulin glargine T2DM ± metformin. Presented at the 74th Scientific Sessions of the American Diabetes Association, San Francisco, CA, USA, 13‐17 June 2014, 1017‐P.
- 27. Defronzo RA, Okerson T, Viswanathan P, Guan X, Holcombe JH, Macconell L. Effects of exenatide versus sitagliptin on postprandial glucose, insulin and glucagon secretion, gastric emptying, and caloric intake: a randomized, cross‐over study. Curr Med Res Opin 2008; 24(10): 2943–2952. [DOI] [PubMed] [Google Scholar]
- 28. Ahrén B, Leguizamo DA, Miossec P, Saubadu S, Aronson R. Efficacy and safety of lixisenatide once‐daily morning or evening injections in type 2 diabetes inadequately controlled on metformin (GetGoal‐M). Diabetes Care 2013; 36(9): 2543–2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bolli GB, Munteanu M, Dotsenko S, et al. Efficacy and safety of lixisenatide once daily versus placebo in patients with type 2 diabetes insufficiently controlled on metformin (GetGoal‐F1). Diabet Med 2014; 31(2): 176–184. [DOI] [PubMed] [Google Scholar]
- 30. Fonseca VA, Alvarado‐Ruiz R, Raccah D, Boka G, Miossec P, Gerich JE. Efficacy and safety of the once‐daily GLP‐1 receptor agonist lixisenatide in monotherapy: a randomized, double‐blind, placebo‐controlled trial in patients with type 2 diabetes (GetGoal‐Mono). Diabetes Care 2012; 35(6): 1225–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pinget M, Goldenberg R, Niemoeller E, Muehlen‐Bartmer I, Guo H, Aronson R. Efficacy and safety of lixisenatide once daily versus placebo in type 2 diabetes insufficiently controlled on pioglitazone (GetGoal‐P). Diabetes Obes Metab 2013; 15(11): 1000–1007. [DOI] [PubMed] [Google Scholar]
- 32. Riddle MC, Forst T, Aronson R, et al. Adding once‐daily lixisenatide for type 2 diabetes inadequately controlled with newly initiated and continuously titrated basal insulin glargine: a 24‐week, randomized, placebo‐controlled study (GetGoal‐Duo 1). Diabetes Care 2013; 36(9): 2497–2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Riddle MC, Aronson R, Home P, et al. Adding once‐daily lixisenatide for type 2 diabetes inadequately controlled by established basal insulin: a 24‐week, randomized, placebo‐controlled comparison (GetGoal‐L). Diabetes Care 2013; 36(9): 2489–2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rosenstock J, Hanefeld M, Shamanna P, et al. Beneficial effects of once‐daily lixisenatide on overall and post‐prandial glycemic levels without significant excess of hypoglycemia in type 2 diabetes inadequately controlled on a sulfonylurea with or without metformin (GetGoal‐S). J Diabetes Complications 2014; 28(3): 386–392. [DOI] [PubMed] [Google Scholar]
- 35. Seino Y, Min KW, Niemoeller E, Takami A. Randomized, double‐blind, placebo‐controlled trial of the once‐daily GLP‐1 receptor agonist lixisenatide in Asian patients with type 2 diabetes insufficiently controlled on basal insulin with or without a sulfonylurea (GetGoal‐L‐Asia). Diabetes Obes Metab 2012; 14(10): 910–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yu PC, Han P, Liu X, et al. Lixisenatide treatment improves glycaemic control in Asian patients with type 2 diabetes mellitus inadequately controlled on metformin with or without sulfonylurea: a randomized, double‐blind, placebo‐controlled, 24‐week trial (GetGoal‐M‐Asia). Diabetes Metab Res Rev 2014; 30(8): 726–735. [DOI] [PubMed] [Google Scholar]
- 37. Cavalot F, Petrelli A, Traversa M, et al. Postprandial blood glucose is a stronger predictor of cardiovascular events than fasting blood glucose in type 2 diabetes mellitus, particularly in women: lessons from the San Luigi Gonzaga Diabetes Study. J Clin Endocrinol Metab 2006; 91(3): 813–819. [DOI] [PubMed] [Google Scholar]


