Figure 1.
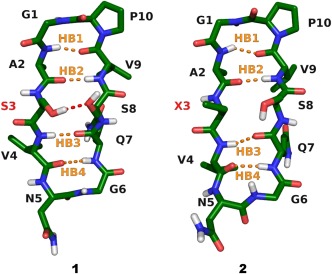
The sequence of cyclic β‐hairpins 1 and 2 is identical apart from an OH to CH3 substitution at the sidechain of the amino acid at position 3. Whereas the folded β‐hairpin conformation of 1 is stabilized by interstrand sidechain to sidechain hydrogen bond of S3 to S8, this interaction is prevented for 2 due to substitution of Ser‐3 to aminobutyric acid (X3). The peptide backbones are shown with carbons in green, nitrogens in blue, oxygens in red, and hydrogens in white. Aliphatic hydrogens are omitted for clarity. Hydrogen bonds are abbreviated as HB1: OV9–HA2, HB2: OA2–HV9, HB3: OQ7–HV4, and HB4: OV4–HQ7 corresponding to the classification used in Table 1.
