Abstract
The design and construction of a dedicated RF coil setup for human brain imaging (1H) and spectroscopy (31P) at ultra‐high magnetic field strength (7 T) is presented. The setup is optimized for signal handling at the resonance frequencies for 1H (297.2 MHz) and 31P (120.3 MHz). It consists of an eight‐channel 1H transmit–receive head coil with multi‐transmit capabilities, and an insertable, actively detunable 31P birdcage (transmit–receive and transmit only), which can be combined with a seven‐channel receive‐only 31P array. The setup enables anatomical imaging and 31P studies without removal of the coil or the patient. By separating transmit and receive channels and by optimized addition of array signals with whitened singular value decomposition we can obtain a sevenfold increase in SNR of 31P signals in the occipital lobe of the human brain compared with the birdcage alone. These signals can be further enhanced by 30 ± 9% using the nuclear Overhauser effect by B 1‐shimmed low‐power irradiation of water protons. Together, these features enable acquisition of 31P MRSI at high spatial resolutions (3.0 cm3 voxel) in the occipital lobe of the human brain in clinically acceptable scan times (~15 min). © 2015 The Authors. NMR in Biomedicine published by John Wiley & Sons Ltd.
Keywords: 31P‐MRS, 31P‐MRSI, spectroscopic imaging, ultra‐high field, RF coil, array coil, multi‐transmit, B1 shimming, WSVD, 7 T
Abbreviations used
- |E|
absolute component of electric field
- |H|
absolute component of magnetic field
- 1H
proton
- 31P
phosphorus‐31
- ATP
adenosine triphosphate
- BC
birdcage
- CH
channel
- CP mode
circularly polarized mode (quadrature driven)
- FOV
field of view
- GPC
glycerophosphocholine
- GPE
glycerophosphoethanolamine
- NOE
nuclear Overhauser effect
- PC
phosphocholine
- PCr
phosphocreatine
- PE
phosphoethanolamine
- Pi
inorganic phosphate
- QL
quality factor in loaded condition
- QU
quality factor in unloaded condition
- ROI
region of interest
- Rx
signal reception
- S12
transmission coefficient
- SAR
specific absorption rate
- SNR
signal‐to‐noise ratio
- Tacq
total acquisition time
- TEM
transverse electromagnetic
- Tx
signal transmission
- VNA
vector network analyzer
- WSVD
whitened singular value decomposition.
Introduction
For many years now, examining the local distribution of metabolites containing phosphorus (31P) atoms in the human brain has been done non‐invasively by in vivo 31P‐MRSI 1. This can give insight in determining or understanding specific metabolic processes, such as energy metabolism or cell membrane turnover. Metabolites of interest for these processes are phosphocreatine (PCr), adenosine triphosphate (ATP), inorganic phosphate (Pi) and the phosphomono‐ and diesters, which can be directly observed in vivo by 31P spectroscopy.
The sensitivity of the 31P nucleus is lower than that of the 1H nucleus (γ 1H/γ 31P = 2.4), but both improve with increasing B 0. Moreover, 1H B 1 homogeneity becomes a real challenge in larger volumes at higher field strengths (≥7 T), but this is less so for 31P. Rodgers et al found a 2.8‐fold increase for 31P‐MRS in the human heart when going from 3 T to 7 T 2. The complexity of spectral patterns of 31P‐MRS is reduced compared with 1H spectra, because fewer metabolites have a 31P nucleus present, and fewer resonances overlap.
Dedicated hardware is needed to detect MR signals from 31P nuclei. A clever design of this hardware can contribute to an improved signal‐to‐noise ratio (SNR) for detection of these signals. RF surface coils, regardless of their operating frequency, have been frequently used for their excellent, albeit spatially constrained, SNR 3. A drawback of RF surface coils is their inhomogeneous B 1 field, requiring complex high‐power adiabatic pulses to homogeneously excite a region of interest (ROI) close to the coil 4. Instead, a fairly uniform flip can be achieved by using volume coils, of course depending on the size of the sample and the operating frequency of the coil. This enables the use of short hard pulses instead of longer adiabatic pulses, as has been shown by Avdievich and Hetherington at 4 T 5. When separating the excitation and reception of MR signals by designing a volume RF coil for excitation and a receive array for acquisition, adiabatic pulse shapes can be avoided and excellent SNR can still be achieved by receiving with small elements 2, 3, 6, 7. The signals from each of these elements must then be combined optimally, which can be achieved with the whitened singular value decomposition (WSVD) method 8, 9.
The 31P RF coil setup needs to be integrated into another RF coil that handles 1H signals. In contrast to clinical MR systems (at B 0 ≤ 3 T), no whole body RF coil is provided as standard within human 7 T systems 10. Therefore, local 1H RF coils must be employed to excite the proton spins and receive their signals, to obtain anatomical images and to be able to optimize the field (B 0 and B 1 shimming). The short wavelengths produce inhomogeneous RF fields; these destructive interferences can coincide within an ROI, decreasing signal intensity at the desired location. When using multiple signal transmission (Tx) coil elements, it is possible to adjust the transmitted RF field by changing input phases and amplitudes of the RF pulses to remove (or shift) these interferences outside the ROI, known as B 1 shimming 11. When multiple coil elements are integrated within a multi‐transmit proton coil it must be assured that local specific absorption rate (SAR) hot‐spots are minimized and remain within guidelines. If by B 1 shimming proton signals of water can be irradiated with low RF power, the nuclear Overhauser effect (NOE) 12 can be used to increase the steady state magnetization of certain 31P nuclei surrounded by water, potentially providing an additional sensitivity to a 31P experiment. This enhancement technique can be used in any organ as long as sufficient power to excite the 1H spins can be delivered to the ROI. Positive NOE enhancements have been reported for prostate and brain 31P MRSI at 7 T 13, 14. Other techniques, such as INEPT, can also be used to enhance signals of the 31P compounds, as has been shown for 3 T 15, 16 and even for 7 T 17, 18. However, as these techniques use rather complex sequences with several inversion pulses, reaching the correct flip angle and not running into SAR restrictions is a major issue. Moreover, T 2 relaxation during long spin evolution times at 7 T could decrease or even negate the polarization gain.
Our aim was to design and construct an integrated coil setup optimized for efficient NOE, uniform 31P spin excitation and high SNR 31P MRS(I) for 7 T. This safety‐validated coil setup should allow optimization of the 1H transmit field (B 1 shimming) and standard 1H imaging as well as uniform excitation and acquisition of 31P spins throughout the human brain, with a possibility of using an additional local receive array and without removing the coil or the patient during an examination. Therefore, we combined an eight‐channel (8‐CH) multi‐transmit head coil (1H) with a newly constructed and insertable actively detunable volume resonator (31P), and developed a 7‐CH receive‐only (Rx) array (31P) that improves SNR locally. Together with optimized signal addition and the possibility to use B 1‐shimmed NOE the sensitivity of local 31P signal detection is maximized.
Materials and Methods
RF coil design and construction
The complete coil setup consists of three parts: an 8‐CH 1H transceiver array (TxRx), an actively detunable 31P volume resonator and a 31P 7‐CH Rx array. Note that the 31P volume resonator can be used as transceiver (without Rx array) and as transmit only (with Rx array).
1H TxRx array
As basis for the complete setup we chose to use an existing 1H head coil designed by Orzada et al. 19. This coil has an octagonal shaped construction and consists of eight intrinsically decoupled microstrip elements with meanders that can be used in multi‐transmit mode 20. Each element was tuned to the resonance frequency of 297.2 MHz and matched to 50 Ω when loaded and with the birdcage (BC) inserted. Cable traps tuned to the resonance frequency of 31P were added to all individual elements.
31P volume resonator (TxRx)
The 31P coil was designed as a BC coil to allow uniform excitation of the 31P signals in the human brain, which should fit inside the 8‐CH head coil. Therefore, we created an eight‐rung, high‐pass design. This design avoids interference of the resonant modes with the 8‐CH head coil, as the highest useful resonance is the dominant mode 21. This eight‐rung design also ensures that the rungs could be positioned exactly between the eight 1H microstrip elements (Fig. 1B).
Figure 1.
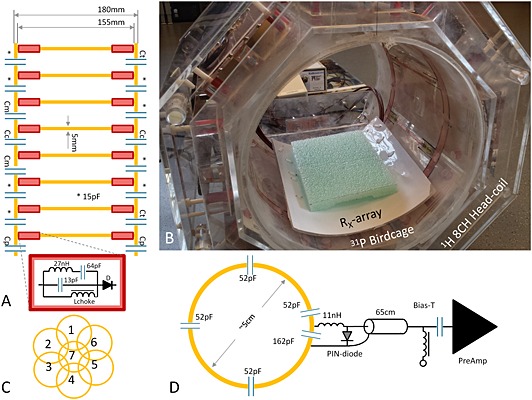
Overview of the complete setup, with detailed representations of the newly designed, detunable 31P BC insert and 31P Rx array. (A) Schematic representation of the eight‐rung high‐pass BC coil; included are details of the tank and detune circuits (red box). Capacitor values used to tune and match the BC coil were C m = 15.6 pF, C t = 13.3 pF, C c = 27 pF and C p = 10 pF. (B) The octagonal‐shaped 8‐CH 1H head coil was used as the basis for the insertable, detunable 31P BC coil, which could host the additional 7‐CH Rx array coil. (C) Schematic configuration of the 7‐CH Rx array. (D) A pictorial overview of a single element of the 7‐CH Rx array, where each adjacent element is decoupled by overlap and by pre‐amplifier decoupling.
The BC was created with copper foil (Parker Chomerics CHO‐FOIL®, Parker Hannifin Corporation, Woburn, MA, USA) attached to a Plexiglas tube (Ø out 24 cm, length 25 cm, thickness 0.5 cm). We enlarged the diameter to 25 cm by opening the tube at one side over the complete length. The opening was fixed by two small Plexiglass parts (l × w 3.2 × 3.2 cm2), which supported the end rings. Two rings of copper foil (width 12.5 mm) were attached to the tube with a spacing of 155 mm. Between the end rings, a total of eight rungs (length 15.5 cm, width 4.5 mm) were placed equidistantly around the tube. A smaller width of the rungs compared with the end rings was chosen to minimize distortion of the 1H field (Fig. 2). The BC was tuned to 120.3 MHz (loaded), by soldering a total capacitance of 14.7 pF in the end rings between the rungs, where a capacitance of 16 pF had been determined with BirdcageBuilder 22. Two tank circuits were added to each rung. Symmetry was preserved by positioning them at both ends. These circuits cause a high impedance at the frequency of 297.2 MHz, while a low impedance is present at 120.3 MHz, hence minimizing the coupling to the 1H elements and optimizing tuning to the 31P Larmor frequency 23. Additionally, a PIN‐diode was placed in series (Fig. 1A) to actively detune the BC during signal acquisition and to enable the use of a local receive array coil. The BC coil had two ports and was driven in circularly polarized (CP) mode (quadrature mode, fixed phase difference of 90° between the two ports). Each port was matched to the characteristic impedance of the MR system (50 Ω) by increasing the matching capacitors to 15.6 pF and by decreasing the capacitors in the opposing end ring to 13.9 pF. The ports were isolated by replacing the capacitors between the matching circuits and the other end ring by 27 pF and by reducing the values of both capacitors that were in the same plane to 8.6 pF. Cable traps tuned to 120.3 MHz to balance the input and to filter common‐mode currents were inserted between the matching circuit and the connection to the MR system.
Figure 2.
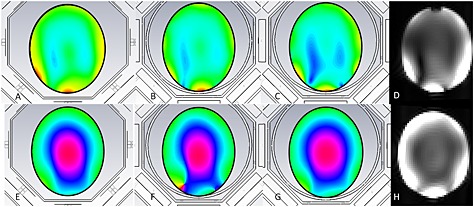
(A–C, E–G) Simulation of proton magnetic field (A–C) and phase (E–G) distribution of the 8‐CH head coil, with and without the 31P BC inserted into the 8‐CH setup. (A, E) The field and phase distributions of the 8‐CH head only, (B, F) the distribution when the BC is inserted (in this case the legs had a width of 12.5 mm), (C, G) the distribution when the width of the legs is reduced to 4.5 mm. (D, H) 1H images of the same phantom with the BC present in the setup; the image in D corresponds to the simulation result as presented in C, hence showing equal field distribution. In H the field distribution is shown with the tank circuits inserted into the BC.
Array coil (Rx only)
A 7‐CH Rx array coil was developed to further improve the SNR of signals arising from 31P compounds. Each circular loop (channel) was made from copper wire (wire diameter 1.25 mm2), having a loop radius of approximately 25 mm and having four symmetrically distributed fixed tuning capacitors (68 pF each). Each loop was matched to 50 Ω (loaded) by placing an additional capacitor (162 pF), which was also part of the active detuning network, in the loop. Part of this active detuning is the parallel positioned PIN‐diode. To create a high impedance at the resonance frequency in the loop, a small inductor of 11 nH was added between the PIN‐diode and the matching capacitor (Fig. 1D). Mutual inductance was minimized by overlapping adjacent loops. Coupling between non‐overlapping loops was reduced by pre‐amplifier decoupling, which transformed the low impedance of the pre‐amplifier to a high impedance at the coil 6, 7.
MR hardware and RF interfaces
All experiments were performed on a 7 T whole body MR system (MAGNETOM 7 T, Siemens Healthcare, Erlangen, Germany). The 8‐CH 1H coil was driven by eight 1 kW RF amplifiers (LPPA 13080 W, Dressler, Germany), where the amplitude and phase of each channel could be altered using a vector modulator, enabling B 1 shimming 11. The TxRx switches with pre‐amps were placed in a separate box at the head of the patient table.
A single 8 kW RF amplifier was used to excite the 31P signal (LPPA 13080 W‐CAN, Dressler, Germany). This signal was divided and phased by a lumped‐element quad‐hybrid to drive the BC in quadrature mode. The 31P receive array was connected to a homebuilt 7‐CH receive interface box optimized for signal acquisition at 120.3 MHz, where in each receive‐path an improved tank circuit 23 was added to trap proton signals. For safety we added to the interface a PIN‐diode breakdown detection circuit, that immediately stops the scanner if a PIN‐diode malfunctions or when a cable is disconnected.
Functionality and safety tests
Bench tests
The BC was tested in TxRx mode and in Tx mode. In the latter case, the coil must be detuned while receiving the MR signal with the local Rx setup. Active tuning was verified by measuring the transmission coefficient (S 12) of the BC, by using two loosely coupled pick‐up probes that were connected to a vector network analyzer (VNA) (R&HZVL3, Rohde & Schwarz, Munich, Germany). This setup was also used to determine the ratio of unloaded‐to‐loaded quality factors (Q U/Q L) 24.
For the receive array, we assessed active detuning, determined the crosstalk between each element, and determined the ratio of unloaded‐to‐loaded quality factors (Q U/Q L) for each element. Active detuning was checked by switching the currents to the PIN‐diode between forward and reverse bias, while measuring the effect of the S 12 response of two pick‐up loops that were weakly coupled to the element under investigation. This method was also used to determine the Q‐factor. Crosstalk between elements was determined by connecting the elements under investigation to the network analyzer, probing signal through one of the elements and receiving with the other. Note that only the element under investigation was tuned and all other coil elements were detuned while making these measurements.
Simulations and RF field measurements
Simulations (CST Studio Suite, CST AG, Darmstadt, Germany) of the setup were made to assess homogeneity of the 1H‐field and to investigate couplings between the two volume coils. Those simulations were performed with and without the BC inserted in the 8‐CH head coil. The receive array was not used in the simulations, but validated with bench measurements. We assumed that existing SAR simulations for the 8‐CH head coil could be maintained when field homogeneity was not altered by the insert 19, 25. We validated these simulations, and the possible additional influence of the 31P Rx array, by assessing the |H|‐ and |E|‐field distributions at both resonances in a phantom filled with head tissue simulating liquid (31P, ε r = 76.5, σ = 0.78 S/m; 1H, ε r = 56.3, σ = 0.98 S/m). Field probes (Schmid & Partner Engineering AG (SPEAG), Zürich, Switzerland, probes H3DV7 & ES3DV2) were used to collect the maps in a sagittal (AP × FH 190 × 190 mm2) and coronal (LR × FH 160 × 190 mm2) plane in the phantom. Data were collected every 10 mm while probing through the complete coil setup with and without the 31P Rx array insert at the desired frequency (1 W). Both planes were positioned along the midline of the phantom (Fig. 3A).
Figure 3.
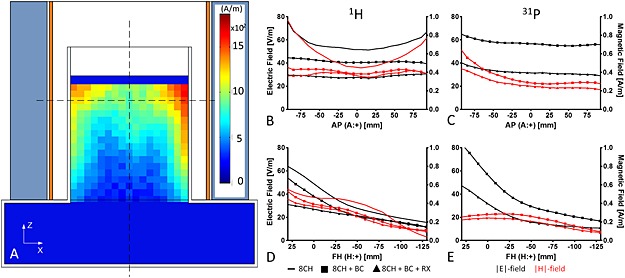
Results of the validation measurements using the field probes. (A) Two‐dimensional visualization of the coil setup surrounding the head–shoulder phantom as it was used to determine the magnitude of the |E| and |H| fields with the field probes. Included is a map of the proton |H| field as it was measured with the 8‐CH head coil only. The phantom was filled with tissue‐simulating fluid, having a conductivity of 0.98 S/m for 1H and 0.78 S/m for 31P, and a permittivity of 56.3 for 1H and 76.5 for 31P. (B–E) Profiles for each individual |E|‐ and |H|‐field measurement (these profiles were taken along the dashed lines (A)) with (B, D) 1H and (C, E) 31P. These measurements were made (i) for the 8‐CH head coil only, (ii) for the 8‐CH head coil with 31P BC inserted and (iii) for the complete setup with receive coil. These measurements were corrected for input power and hence are directly comparable to those of Reference 25. Note that |E| and |H| fields decrease when more components are present.
31P MR of phantom and volunteers
The complete functionality of the coil setup was checked with the following tests: (i) 90° flip angle calibration of the BC, (ii) determination of the SNR increase of the 31P signal when acquiring data with the Rx array instead of the BC, (iii) obtaining the noise correlation matrix of the 7‐CH Rx array, and (iv) 1H B 1 + shimming with all 31P components present. For these tests a cylindrical phantom (diameter 16 cm and volume ~ 5 L) filled with inorganic phosphate (Pi) (30 mM) (and 2% agar) was used. For an additional in vivo test two healthy volunteers (males, 24 years and 26 years) were scanned after giving written informed consent.
A repeated slice selective pulse acquire sequence (6 ms excitation pulse (sinc), T R = 15 s, single shot) selecting a 40 mm thick axial slice through the center of the coil was used for flip angle calibration, where the RF amplitude of the excitation pulse was varied to find the maximum amplitude of the peak present in the phantom (Pi), which approximates a 90° excitation.
Two 3D MRSI data sets of the same cylindrical phantom were obtained to determine the SNR performance of the 31P coils. In both experiments, we excited the 31P signal using the BC, but acquired the signal with the BC in one, and acquired the signals with the 31P Rx array in the other experiment. Signals from the individual receive channels were combined using the WSVD combination method 8, comprising (i) a very short noise prescan that is used to decorrelate the single‐element spectra (or FIDs) and (ii) computation of the maximum likelihood combined spectrum. The Rx array was not present in the first test with the BC alone, thus before the second test the phantom was accurately repositioned after the Rx array was inserted. The 3D pulse–acquire MRSI experiment had the following parameters: T R/acquisition delay 1000/0.1 ms, flip angle 40°, hard pulse duration 0.3 ms, field of view (FOV) 240 × 240 × 200 mm3, matrix 12 × 12 × 8, elliptical k‐space acquisition with 100% Hamming filter, vector size 1024 and T acq 5 min 15 s. The spectra from the MRSI data were fitted with Metabolite Report, a work in progress package from Siemens Healthcare (Erlangen, Germany). Metabolite Report performs automated, prior‐knowledge‐based, complex fitting in the time domain and has previously been applied to 7 T 31P spectroscopy data of the prostate 13. Images of the fitted Pi signal were created by interpolating its initial 12 × 12 × 8 matrix to a 256 × 256 × 8 matrix. They were then convoluted by a disk‐shaped kernel and masked. These images were then used to determine the local signal gain when using the Rx array as compared with the BC. An additional examination without RF excitation pulses was performed to obtain the noise correlation matrix.
In an in vivo study we investigated the ability to use B 1 shimming and the ability to acquire 3D 31P MRSI in the human brain with and without NOE enhancement on a single volunteer (24 years) (T R/acquisition delay 1500/0.10 ms, flip angle 45°, matrix 12 × 12 × 8, FOV 240 × 240 × 240 mm3, pulse length 0.3 ms, elliptical k‐space acquisition with 100% Hamming filter, T acq = 7 min 48 s). A high‐resolution 3D 31P MRSI using NOE enhancement with an approximated true voxel size of 3.0 cm3 was acquired from the second volunteer (26 years). This time data was collected with the Rx array, providing a reduced FOV. Parameters used for this sequence were T R/acquisition delay 500/0.10 ms, flip angle 30°, FOV 140 × 140 × 100 mm3, matrix 14 × 14 × 10, NSA = 6 and T acq 15 min 2 s; all other parameters were kept the same. As the T R is reduced and we want the maximum signal per unit time, we reduced the flip angle to the Ernst angle for PCr (30°) with T 1 = 3.4 ± 0.3 s 14. In each examination the NOE enhancement was generated by saturating the water signals (γB 1 = 30 Hz) using the wideband alternating‐phase low‐power technique for zero residual splitting (WALTZ‐4, technique originally intended for decoupling) 26 during the full T R, except during the 204 ms of signal acquisition. Other examinations within the protocol were the same for both volunteers. After B 0 and B 1 shimming the whole brain, a 3D T 1‐weighted image was acquired with an MPRAGE (magnetization prepared rapid acquisition gradient echo) pulse sequence. For this sequence the following parameters were used: T R/T I/T E 2500/1100/1.270 ms, resolution 1 mm3, T acq 3 min 58 s. Then we calibrated the flip angle for 31P, and we optimized the B 1 shim for the occipital lobe.
Results
Bench tests
RF coils
The ratio of unloaded‐to‐loaded quality factors (Q U/Q L) for the BC was 110/30 = 3.7. For the receive array it was (155 ± 20)/(63 ± 5) = 2.7 ± 0.3, with a range of 124–181 for Q U and 57–69 for Q L.
Simulations and RF field measurements
Simulations were used to determine the influence of the 31P BC at the 1H field when it is inserted into the 8‐CH head coil. These showed that standard 12.5 mm wide copper tape for the legs created a signal void a few centimeters inside the phantom: coinciding with this void was a phase singularity (Fig. 2B, F). Simulations showed that the signal void and the phase singularity could be removed when the width of the legs was reduced to 4.5 mm (Fig. 2C, G), although with reduced sensitivity. The sensitivity increased and could be related to the field intensity of the 8‐CH head‐coil without BC when the tank circuitry was added to the BC (Fig. 2H).
The field probe measurements visualized the field distribution for both nuclei. For the 1H field in general, we concluded that the overall field distribution in the phantom was comparable to that from the reference coil 25, but that amplitudes in the center had been decreased for this new setup. This was shown by the coronal map of the |H| field at 297 MHz for the 8‐CH head coil only (Fig. 3A). Note that the 8‐CH head coil was tuned and matched to the resonance frequency with the BC inserted.
The proton |H|‐ and |E|‐field amplitudes at both resonances for the coil assembly in different configurations (Fig. 3) illustrated that the amplitudes of both field types decreased with the BC inserted. Signals did not decrease further with the Rx coil present. Moreover, the stronger decrease in |E| field (causing SAR deposition) compared with the |H| field confirmed that it was safe to use this coil assembly with the original 8‐CH safety margins.
Comparing these results with the 8‐CH proton setup without any additional coils 19, 25 enabled us to use the same maximal permissible input power, since the magnitude of the electric field is reduced after insertion of the additional components, providing an even greater safety margin in the case of the new 1H coil assembly. For 31P the maximum permissible input power was calculated based on a worst‐case approximation (losses in the feeding network and RF coil were neglected) for the head‐averaged SAR according to the rationale given in the IEC 60601‐2‐33 for volume transmit coils: P max 31P = SARhead,limit m head.
31P MR of phantom
Calibration of the RF power to reach a 90° flip angle for the 31P signals in a phantom as well as in the human brain showed that the maximum signal intensity coincided with a reference B 1 + amplitude of 29 μT. This resulted in a maximum achievable B 1 + amplitude for 31P of approximately 56 μT for this coil setup (on average this value was a little lower, 45–50 μT).
The noise correlation matrix (Fig. 4) confirmed that coupling between all elements was minimized as a result of overlap and pre‐amplifier decoupling. The average noise correlation over all elements was 10 ± 7%, with its maximum of 23% found between elements 3 & 6.
Figure 4.
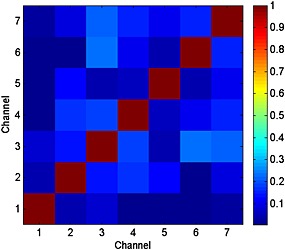
Noise correlation matrix of the 7‐CH Rx array. All channels are properly decoupled, because noise does not correlate significantly. The average noise correlation over all elements was 10 ± 7%, with its maximum of 23% found between elements 3 and 6.
As expected, the SNR increased substantially when the Rx array was used for signal acquisition as compared with the BC. An increase in SNR was visible up to 7 cm inside the phantom, with a sevenfold increase up to 2 cm inside the phantom (Fig. 5).
Figure 5.
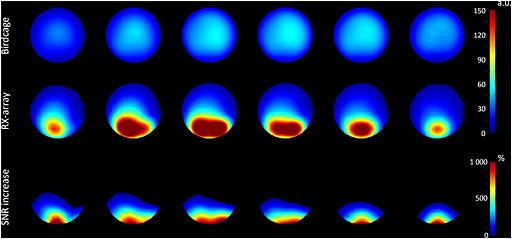
Interpolated SNR images of a spherical phantom containing 30 mM inorganic phosphate. The images were obtained with the BC coil (top row) and with the local Rx array (middle row). The gain in SNR is more than sevenfold close to the receive array. Also note the uniform B 1 field when data was solely acquired with the BC.
31P MR of volunteers
The in vivo experiments were successful. We could obtain detailed T 1‐weighted images with the 8‐CH 1H head coil driven in CP+ mode (Fig. 6, background) and were able to shim the 1H B 1 + field (Fig. 6A–C). This allowed us to maximally enhance 31P signals using a B 1‐shimmed setting for specified regions. For the whole brain, we plotted the metabolite map of PCr when measured with and also without NOE enhancement (Fig. 6D, E). An enhancement map showed the signal increase, expressed as a percentage (Fig. 6F); the main features of this enhancement map coincided with the 1H B 1 + field (Fig. 6C). The overall enhancement of the PCr signal was 30 ± 9%.
Figure 6.
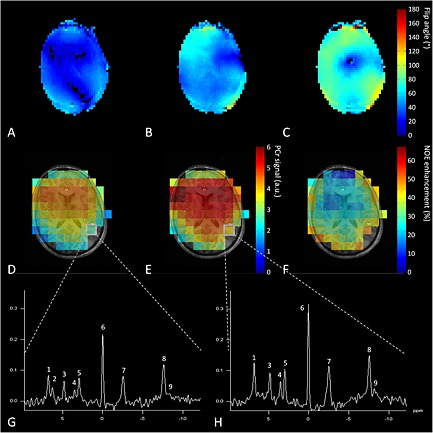
(A–C) Results of in vivo experiment using the complete setup showing different B 1 + maps of the 1H field: (A) in CP− mode, (B) homogenized for the complete brain, and (C) locally optimized for occipital lobe. Showing the ability to use B 1 shimming with the coil setup. Background images were acquired with the 8‐CH 1H head coil driven with global B 1 optimization, showing fairly homogeneous field distribution. (D–F) 31P spins were excited and signal was acquired with the BC (D) without and (E) with NOE enhancement of PCr; (F) the global enhancement map. (G, H) An example of 31P spectra taken from the same voxel (G) without NOE enhancement and (H) with NOE enhancement. Spectra were obtained in 7 min 48 s with an approximate voxel size of 38 cm3. The metabolites present in these spectra are (1) phosphoethanolamine (PE), (2) phosphocholine (PC), (3) Pi, (4) glycerophosphoethanolamine (GPE), (5) glycerophosphocholine (GPC), (6) PCr, (7) γ‐ATP, (8) α‐ATP and (9) NADH.
When acquiring signals with the local receive array, we could reduce the FOV and hence increase spatial resolution. Combined with local NOE enhancement and an optimized signal combination method (i.e. WSVD combination), we obtained 31P spectra from relatively small voxels (3.0 cm3) in 15 min, with narrow linewidths for PCr (9.6 ± 1.9 Hz) and high SNR (17.8 ± 3.7) (Fig. 7), up to 5 cm into the occipital lobe.
Figure 7.
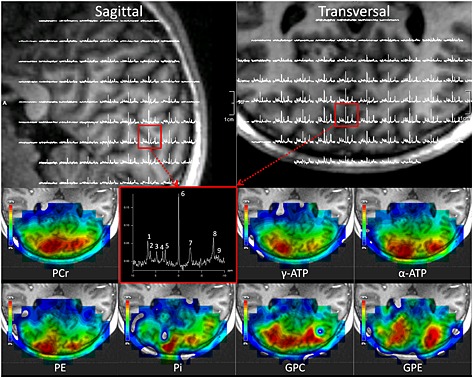
High‐resolution 3D MRSI using all available options to increase SNR. The signals were enhanced using NOE and were received with the local receive array. Note the exquisite quality of the spectra. Top left, a sagittal view of the human brain overlapped with 31P spectra as acquired with the receive array. Top right, a transversal view of the human brain also overlapped with a spectral map covering the occipital lobe. Bottom two rows, metabolite maps of seven different 31P compounds. The colors show the distribution of the metabolites (scaled 0–100%) represented by the fitted integral. Note that in only two voxels were low‐SNR metabolites (Pi and GPC) fitted incorrectly within the FOV of the array coil. Moreover, note the difference in spatial distributions of the different metabolites, which arise not only from the B 1‐receiving profile (anterior–posterior), but also from differences between gray and white matter (left–right). A spectrum with high quality (3 cm3 voxel), as acquired in 15 min in the occipital lobe (red box), is shown too. Not shown in this spectrum is the β‐ATP, as the bandwidth of the pulse to properly excite this metabolite was insufficient. (1) PE, (2) PC, (3) Pi, (4) GPE, (5) GPC, (6) PCr, (7) γ‐ATP, (8) α‐ATP and (9) NADH.
The SAR for the experiments with NOE enhancement was evaluated using the real‐time SAR monitoring system 27. The time‐averaged total input power for these experiments (not incorporating coil losses) was 10.9 W for 1H and 2.2 W for 31P.
Discussion
In this work, we have described the design, construction, and safety validation of a dedicated coil setup for 7 T containing three main components: an 8‐CH multi‐transmit 1H head coil, an insertable and actively tunable BC coil for 31P and a local 7‐CH 31P Rx array. The setup featured multi‐transmit capabilities on the 1H channels, volume excitation for 31P, and a large local SNR improvement close to the receive array. This increase in sensitivity in combination with the limited FOV of local receive coils enabled 31P MRSI at higher spatial resolution, and may be used to reduce scan time.
The tuning and matching of the 8‐CH 1H head coil had to be adapted, because its initial tuning frequency dropped below the resonance frequency of the system. Therefore we tuned it to the correct frequency and adapted the matching to become 50 Ω (loaded) with the BC present. Despite this adaption, we noticed a reduction in sensitivity of the 1H signal, which is probably caused by the large quantity of conductive material present in the BC itself. Although this might block part of the signal, as reported for high‐density receive arrays 28, we did not observe a change in the shape of the field distributions in the phantom measurements.
Although not shown here, it is worth noting that the homogeneity of the BC was hardly altered by the addition of the local Rx array. Each element in the receive array was actively detuned, meaning that a current was used to forward bias a parallel positioned PIN‐diode, bringing the element off resonance during BC transmit. We added no additional safety features, such as a second trap with passive diodes 29 or an RF fuse, to the individual loops in order to avoid a possible decrease in SNR. Instead, we considered (and verified) that, should a parallel‐positioned PIN‐diode break down, a short circuit would be created and hence the coil element would be detuned, which makes it intrinsically safe. However, this feature does not detect a moment when no current flows through the PIN‐diode (e.g. when a cable is disconnected). Therefore, we added a current sensing circuit inside the interface, which activates and stops the scanner immediately if no current goes to the PIN‐diodes.
The SNR increase by exciting the spins via the BC and receiving their signal with the array coil may have been biased, as the BC for reception may have had degraded performance. The PIN‐diodes for tuning the BC were positioned in series within the legs: when forward biased (tuned) they have a low additional series resistance; when the PIN‐diodes are reverse biased (detuned) this resistance will increase significantly. However, the coil has a relatively high Q U (110), and when the Q U/Q L ratio is above 3.5 tissue loading dominates over coil losses. Hence, the introduction of PIN‐diodes in the BC did not degrade its performance in the first place.
Increasing SNR by separating Rx and Tx surface coils for 31P spectroscopy has been reported before 30, 31, where Avdievich and Hetherington were the first to design and develop a homogeneous transmit volume coil 5, which was the basis for our work. Their coil was designed as an actively detunable double‐tuned transverse electromagnetic (TEM) head coil for 4 T. The two setups have similar properties and both can provide anatomical reference images and 31P MRSI of the patient without removal of the coil or the patient. The advantage of our design over theirs is the ability to use B 1 shimming. Moreover, the size of the individual elements of the Rx 31P array in our design was smaller, resulting in a local increase in SNR.
It was shown by Lei et al. that signal from PCr in the human brain can be increased by 24.3 ± 1.6% using NOE 14. However, this effect depends on the amplitude and distribution of the 1H B 1 field (Fig. 6C, F) and can therefore vary spatially 13. This principle can be exploited to enhance specific regions. If NOE is used, it is essential to reach a threshold 1H B 1 + value in a certain area to achieve maximum NOE enhancement. An additional 1H B 1 + map is therefore needed to assess the locations where the threshold B 1 + was reached. 31P B 1 + does not affect the NOE variation. With the current coil setup, an overall enhancement of 30 ± 9% for the PCr signal could be reached in the human brain. This higher enhancement compared with the data of Lei et al. may be related to the proton irradiation power of this coil and our choice of the WALTZ‐4 decoupling strategy.
Although the amplitude of the 1H signals was reduced with the 31P BC inserted, we were able to optimize the B 1 + field and use this to enhance signals. Moreover, the irradiation of the water spins with low RF power led to a time‐averaged maximum input power of 10.9 W. Assuming the weight of an average head to be approximately 5 kg, this leads to a worst‐case head‐averaged SAR of 2.18 W/kg, which is already well below the IEC‐defined limit of 3.2 W/kg for the head. Within this approximation losses inside the coil (e.g. feed network, coil elements, etc.) are also present, thus the head‐averaged SAR would be reduced even further. Furthermore, when using phased array coils for exciting the MR signal instead of volume resonators, SAR deposition can be reduced significantly (up to 50%) while optimizing the B 1 + distribution, as has been shown by van den Bergen et al. in the pelvic area 32. Although this was investigated at 3 and 7 T with a TEM coil, it was shown by Wang et al. that SAR levels are lower in microstrip coil designs at 7 T 33. These findings support the usage of this 8‐CH multiple‐array antenna. Moreover, the meander structures in the element decrease intrinsic coupling of the elements, and reduce the magnitude of the electric field at the ends of the elements 20. A striking fact from the same study is that SAR levels for BC coils, such as our new design for 31P, were higher at 7 T. Note that we optimized this BC coil for a lower frequency (120.3 MHz), and when using volume coils at these frequencies the head‐averaged SAR is usually the most critical aspect.
Most studies on 31P in the human brain at 7 T have been using surface coils to excite and receive the MR signal 14, 34, 35, except for that by Zhu et al. 36, who used a CP dual‐frequency volume RF coil. Although surface coils have a higher SNR, their B 1 fields are inhomogeneous, hence the flip angle becomes position dependent should one use a hard pulse for excitation. By using adiabatic pulses these limitations can be circumvented, but at a cost of markedly increased pulse duration and concomitant increase in SAR deposition. Using the homogenous volume resonator present here allowed us to avoid SAR demanding adiabatic pulses, but rather use short (0.3 ms) hard pulses that have sufficient bandwidth to excite the spectrum (except β‐ATP). Note that Zhu et al. were not able to use these in their study 36.
In conclusion, the developed coil setup can be used to excite 1H and 31P signals at an ultra‐high field strength of 7 T. 31P signals can be acquired with an increased SNR by exploiting NOE enhancement and by receiving the signals with a local receive array.
Acknowledgements
We thank M.J. van Uden and A. Veltien for the extensive discussions about safety, design, construction, and implementation of coilfiles, E.W.J. Philips for implementing the WSVD code into the pulse sequence, Y. Noureddine for his help setting up the field probe measurements, and Elisabeth Weiland from Siemens Healthcare for providing Metabolite Report. Part of this work was supported by Grant 243115 from the European Research Council under the European Community's Seventh Framework Programme (FP7/2007–2013). C.T. Rodgers is funded by the Wellcome Trust and the Royal Society (Grant Number 098436/Z/12/Z).
van de Bank, B. L. , Orzada, S. , Smits, F. , Lagemaat, M. W. , Rodgers, C. T. , Bitz, A. K. , and Scheenen, T. W. J. (2015) Optimized 31P MRS in the human brain at 7 T with a dedicated RF coil setup. NMR Biomed., 28: 1570–1578. doi: 10.1002/nbm.3422.
References
- 1. Hugg JW, Matson GB, Twieg DB, Maudsley AA, Sappey‐Marinier D, Weiner MW. Phosphorus‐31 MR spectroscopic imaging (MRSI) of normal and pathological human brains. Magn. Reson. Imaging 1992; 10(2): 227–243. [DOI] [PubMed] [Google Scholar]
- 2. Rodgers CT, Clarke WT, Snyder C, Vaughan JT, Neubauer S, Robson MD. Human cardiac 31P magnetic resonance spectroscopy at 7 Tesla. Magn. Reson. Med. 2014; 72(2): 304–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wright SM, Wald LL. Theory and application of array coils in MR spectroscopy. NMR Biomed. 1997; 10(8): 394–410. [DOI] [PubMed] [Google Scholar]
- 4. Tannus A, Garwood M. Adiabatic pulses. NMR Biomed. 1997; 10(8): 423–434. [DOI] [PubMed] [Google Scholar]
- 5. Avdievich NI, Hetherington HP. 4 T actively detuneable double‐tuned 1H/31P head volume coil and four‐channel 31P phased array for human brain spectroscopy. J. Magn. Reson. 2007; 186(2): 341–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Roemer PB, Edelstein WA, Hayes CE, Souza SP, Mueller OM. The NMR phased array. Magn. Reson. Med. 1990; 16(2): 192–225. [DOI] [PubMed] [Google Scholar]
- 7. Keil B, Wald LL. Massively parallel MRI detector arrays. J. Magn. Reson. 2013; 229: 75–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rodgers CT, Robson MD. Receive array magnetic resonance spectroscopy: whitened singular value decomposition (WSVD) gives optimal Bayesian solution. Magn. Reson. Med. 2010; 63(4): 881–891. [DOI] [PubMed] [Google Scholar]
- 9. Rodgers CT, Robson MD. Coil combination for receive array spectroscopy: are data‐driven methods superior to methods using computed field maps? Magn. Reson. Med. 2015. DOI:10.1002/mrm.25618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Vaughan JT, Snyder CJ, DelaBarre LJ, Bolan PJ, Tian J, Bolinger L, Adriany G, Andersen P, Strupp J, Ugurbil K. Whole‐body imaging at 7 T: preliminary results. Magn. Reson. Med. 2009; 61(1): 244–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Metzger GJ, Snyder C, Akgun C, Vaughan T, Ugurbil K, Van de Moortele PF. Local B 1 + shimming for prostate imaging with transceiver arrays at 7 T based on subject‐dependent transmit phase measurements. Magn. Reson. Med. 2008; 59(2): 396–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Luyten PR, Bruntink G, Sloff FM, Vermeulen JW, van der Heijden JI, den Hollander JA, Heerschap A. Broadband proton decoupling in human 31P NMR spectroscopy. NMR Biomed. 1989; 1(4): 177–183. [DOI] [PubMed] [Google Scholar]
- 13. Lagemaat MW, Maas MC, Vos EK, Bitz AK, Orzada S, Weiland E, van Uden MJ, Kobus T, Heerschap A, Scheenen TW. 31P MR spectroscopic imaging of the human prostate at 7 T: T1 relaxation times, Nuclear Overhauser Effect, and spectral characterization. Magn. Reson. Med. 2014; 73(3): 909–920. DOI:10.1002/mrm.25209. [DOI] [PubMed] [Google Scholar]
- 14. Lei H, Zhu XH, Zhang XL, Ugurbil K, Chen W. In vivo 31P magnetic resonance spectroscopy of human brain at 7 T: an initial experience. Magn. Reson. Med. 2003; 49(2): 199–205. [DOI] [PubMed] [Google Scholar]
- 15. Klomp DW, Wijnen JP, Scheenen TW, Heerschap A. Efficient 1H to 31P polarization transfer on a clinical 3 T MR system. Magn. Reson. Med. 2008; 60(6): 1298–1305. [DOI] [PubMed] [Google Scholar]
- 16. Wijnen JP, Scheenen TW, Klomp DW, Heerschap A. 31P magnetic resonance spectroscopic imaging with polarisation transfer of phosphomono‐ and diesters at 3 T in the human brain: relation with age and spatial differences. NMR Biomed. 2010; 23(8): 968–976. [DOI] [PubMed] [Google Scholar]
- 17. van der Kemp WJ, Boer VO, Luijten PR, Klomp DW. Increased sensitivity of 31P MRSI using direct detection integrated with multi‐echo polarization transfer (DIMEPT). NMR Biomed. 2014; 27(10): 1248–1255. [DOI] [PubMed] [Google Scholar]
- 18. van der Kemp WJ, Boer VO, Luijten PR, Wijnen JP, Klomp DW. Increase in SNR for 31P MR spectroscopy by combining polarization transfer with a direct detection sequence. Magn. Reson. Med. 2012; 68(2): 353–357. [DOI] [PubMed] [Google Scholar]
- 19. Orzada S, Kraff O, Brote I, Schäfer LC, Bahr A, Bolz T, Maderwald S, Ladd ME, Bitz AK. 8‐channel transmit/receive head coil for 7 T human imaging using intrinsically decoupled strip‐line elements with meanders. Proc. Int. Soc. Magn. Reson. Med. 2009; 17: 3010. [Google Scholar]
- 20. Rietsch SHG, Quick HH, Orzada S. Impact of different meander sizes on the RF transmit performance and coupling of microstrip line elements at 7 T. Med. Phys. 2015; 42(8): 4542–4552. [DOI] [PubMed] [Google Scholar]
- 21. Leifer MC. Resonant modes of the birdcage coil. J. Magn. Reson. 1997; 124(1): 51–60. [Google Scholar]
- 22. Chin CL, Collins CM, Li SH, Dardzinski BJ, Smith MB. BirdcageBuilder: design of specified‐geometry birdcage coils with desired current pattern and resonant frequency. Concepts Magn. Reson. 2002; 15(2): 156–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Meyerspeer M, Roig ES, Gruetter R, Magill AW. An improved trap design for decoupling multinuclear RF coils. Magn. Reson. Med. 2013; 72(2): 584–590. DOI:10.1002/mrm.24931. [DOI] [PubMed] [Google Scholar]
- 24. Hoult DI, Lauterbur PC. The sensitivity of the zeugmatographic experiment involving human samples. J. Magn. Reson. 1979; 34(2): 425–433. [Google Scholar]
- 25. Bitz A, Kraff O, Orzada S, Maderwald S, Brote I, Johst S, Ladd M. Assessment of RF safety of transmit coils at 7 Tesla by experimental and numerical procedures. Proc. Int. Soc. Magn. Reson. Med. 2011; 19: 490. [Google Scholar]
- 26. Shaka AJ, Keeler J, Freeman R. Evaluation of a new broadband decoupling sequence: WALTZ‐16. J. Magn. Reson. 1983; 53(2): 313–340. [Google Scholar]
- 27. Bitz A, Brote I, Orzada S, Kraff O, Maderwald S, Quick H, Yazdanbakhsh P, Solbach K, Bahr A, Bolz T, Wickdow K, Schmitt F, Ladd M. An 8‐channel add‐on RF shimming system for whole‐body 7 Tesla MRI including real‐time SAR monitoring. Proc. Int. Soc. Magn. Reson. Med. 2009; 17: 4767. [Google Scholar]
- 28. van de Bank BL, Voogt IJ, Italiaander M, Stehouwer BL, Boer VO, Luijten PR, Klomp DW. Ultra high spatial and temporal resolution breast imaging at 7 T. NMR Biomed. 2012; 26(4): 367–375. DOI:10.1002/nbm.2868. [DOI] [PubMed] [Google Scholar]
- 29. Edelstein WA, Hardy CJ, Mueller OM. Electronic decoupling of surface‐coil receivers for NMR imaging and spectroscopy. J. Magn. Reson. 1986; 67(1): 156–161. [Google Scholar]
- 30. Hardy CJ, Bottomley PA, Rohling KW, Roemer PB. An NMR phased array for human cardiac 31P spectroscopy. Magn. Reson. Med. 1992; 28(1): 54–64. [DOI] [PubMed] [Google Scholar]
- 31. Lee RF, Giaquinto R, Constantinides C, Souza S, Weiss RG, Bottomley PA. A broadband phased‐array system for direct phosphorus and sodium metabolic MRI on a clinical scanner. Magn. Reson. Med. 2000; 43(2): 269–277. [DOI] [PubMed] [Google Scholar]
- 32. van den Bergen B, Van den Berg CA, Bartels LW, Lagendijk JJ. 7 T body MRI: B 1 shimming with simultaneous SAR reduction. Phys. Med. Biol. 2007; 52(17): 5429–5441. [DOI] [PubMed] [Google Scholar]
- 33. Wang CS, Shen GX. B 1 field, SAR, and SNR comparisons for birdcage, TEM, and microstrip coils at 7 T. J. Magn. Reson. Imaging 2006; 24(2): 439–443. [DOI] [PubMed] [Google Scholar]
- 34. Chmelík M, Just Kukurová I, Gruber S, Krššák M, Valkovič L, Trattnig S, Bogner W. Fully adiabatic 31P 2D‐CSI with reduced chemical shift displacement error at 7 T — GOIA‐1D‐ISIS/2D‐CSI. Magn. Reson. Med. 2013; 69(5): 1233–1244. [DOI] [PubMed] [Google Scholar]
- 35. Lu M, Chen W, Zhu X‐H. Field dependence study of in vivo brain 31P MRS up to 16.4 T. NMR Biomed. 2014; 27(9): 1135–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zhu XH, Qiao H, Du F, Xiong Q, Liu X, Zhang X, Ugurbil K, Chen W. Quantitative imaging of energy expenditure in human brain. Neuroimage 2012; 60(4): 2107–2117. [DOI] [PMC free article] [PubMed] [Google Scholar]


