Table 6.
Commercially available building blocking blocks for introduction of principle PTMs
| PTM | Introduction | Comments | Reference |
|---|---|---|---|
| Phosphorylation Ser/Thr | |||
|
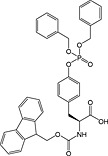
|
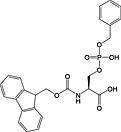
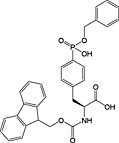
Fmoc‐Ser(PO(OBzl)OH)‐OH 17 | Best coupled using imminium‐based reagents 135 | Wakamiya et al., 1994 136 |
|
|
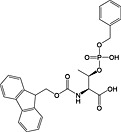
Fmoc‐Thr(PO(OBzl)OH)‐OH 18 | Best coupled using imminium‐based reagents 135 | White and Beythien, 1996 137 |
|
|
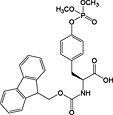
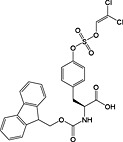
Fmoc‐Tyr(PO(OMe)2)‐OH 19 | Compatible with all coupling methods; monodemethylated by piperidine; requires TMSBr/TFA for side‐chain deprotection | Kitas et al., 1989 138 |
|
|
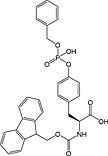
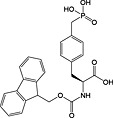
Fmoc‐Tyr(PO(OBzl)OH)‐OH 20 | Best coupled using imminium‐based reagents 154 | White and Beythien, 1996 137 |
|
|
Fmoc‐Tyr(PO(OBzl)2)‐OH 21 | Compatible with all coupling methods; monodebenzylated by piperidine | Perich and Reynolds, 1991 139 |
|
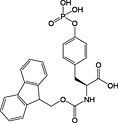
|
Fmoc‐Tyr(PO3H2)‐OH 22 | Best coupled using imminium‐based reagents; issues with pyrophosphate formation 143 | Ottinger et al., 1993 140 |
|
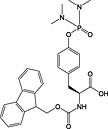
|
Fmoc‐Tyr(PO(NMe2)2)‐OH 23 | Compatible with all coupling methods; deprotected with TFA/water (9 : 1) | Chao et al., 1995 141 |
|
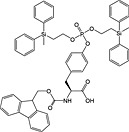
|
Fmoc‐Tyr(PO(OMDPSE)2)‐OH 24 | Compatible with all coupling methods; MDPSE groups removed with TFA | Chao et al., 1994 142 |
|
|
Fmoc‐Ppa(Bzl)‐OH 25 | Best coupled using imminium‐based reagents | Chauhan et al., 2007 143 |
|
|
Fmoc‐Pmp‐OH 26 | Best introduced with HATU/DIPEA coupling | Marseigne et al., 1988 144 |
|
|
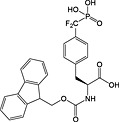
Fmoc‐F2Pmp‐OH 27 | Best introduced with HATU/DIPEA coupling | Gordeev et al., 1994 145 |
| Sulfation Tyr | |||
|
|
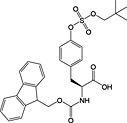
Fmoc‐Tyr(SO3nP)‐OH 28 | Neopentyl ester is stable to TFA; cleaved with sodium azide/DMSO or aq. ammonium acetate | Simpson and Widlanski, 2006 [146, 147] |
|
|
Fmoc‐Tyr(SO3DCV)‐OH 29 | DCV ester stable to TFA; DCV cleaved by Zn/AcOH reduction | Ali and Taylor, 2009 148, 149 |
| Methylation Arg | |||
|
|
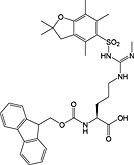
Fmoc‐Arg(Me,Pbf)‐OH 30 | For introduction of monomethyl arginine | White, 2006 150 |
|
|
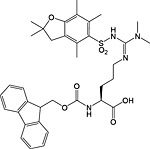
Fmoc‐ADMA(Pbf)‐OH 31 | For introduction of asymmetric dimethylarginine | White et al., 2006 150 |
|
|
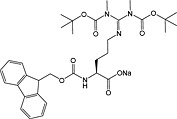
Fmoc‐SDMA(Boc2)‐ONa 32 | For introduction of symmetric dimethylarginine | White et al., 2006 150 |
| Methylation Lys | |||
|
|
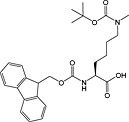
Fmoc‐Lys(Me,Boc)‐OH 33 | For introduction of monomethyl lysine | |
|
|
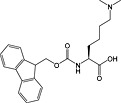
Fmoc‐Lys(Me2)‐OH 34 | For introduction of dimethyl lysine, basic side chain can promote Fmoc loss and double insertions during synthesis 151 | |
|
|
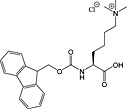
Fmoc‐Lys(Me3Cl)‐OH 35 | For introduction of trimethyl lysine | |
| Citrullation | |||
|
|
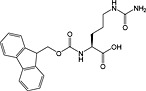
Fmoc‐citrulline‐OH 36 | For introduction of citrullation | |
| Glycosylation Asn | |||
|
|
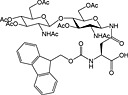
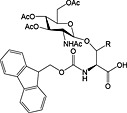
Fmoc‐Asn(β‐d‐GlcNAc(Ac)3)‐OH 37 | Building block for introduction of monosaccharide fragment of N‐linked glycoproteins | Meldal and Bock, 1990 152 |
|
|
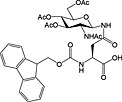
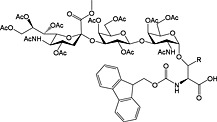
Fmoc‐Asn(β‐d‐GlcNAc(Ac)3‐(1‐4)‐β‐d‐GlcNAc(Ac)2)‐OH 38 | Building block for introduction of chitobiose fragment of N‐linked glycoproteins | Meinjohanns et al., 1998 153 |
| Glycosylation Ser (R = H)/Thr (R = Me) | |||
|
|
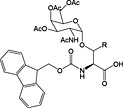
Fmoc‐Ser/Thr(α‐d‐GlnNAc(Ac)3)‐OH 39 | Building block for introduction of Tn antigen oligosaccharide fragment | Paulsen and Adermann, 1989 154 |
|
|
Fmoc‐Ser/Thr(β‐d‐Gal(Ac)4‐(1‐3) α‐d‐GlnNAc(Ac)2)‐OH 40 TF antigen | Building block for introduction of TF antigen oligosaccharide fragment | Irazoqui et al., 1999 155 |
|
|
Fmoc‐Ser/Thr(sialylOMe(Ac)4‐(1‐6)‐α‐d‐GlnNAc(Ac)2)‐OH 41 STn antigen | Building block for introduction of STn antigen oligosaccharide fragment | Liebe and Kunz, 1997 156 |
|
|
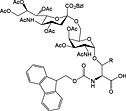
Fmoc‐Ser/Thr(sialylOMe(Ac)4‐(1‐3)‐β‐d‐Gal(Ac)3‐(1‐3) α‐d‐GlnNAc(Ac)2)‐OH 42 STF antigen | Building block for introduction of STn antigen oligosaccharide fragment | Komba et al., 1999 157 |
|
|
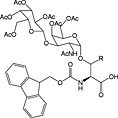
Fmoc‐Ser/Thr(β‐d‐GlcNAc(Ac)3)‐OH 43 | Building block for introduction of β‐GlcNAc modification; building blocks tend to racemise 158 | Arsequell et al., 1994 159 |