Figure 7. Hypothetical Model.
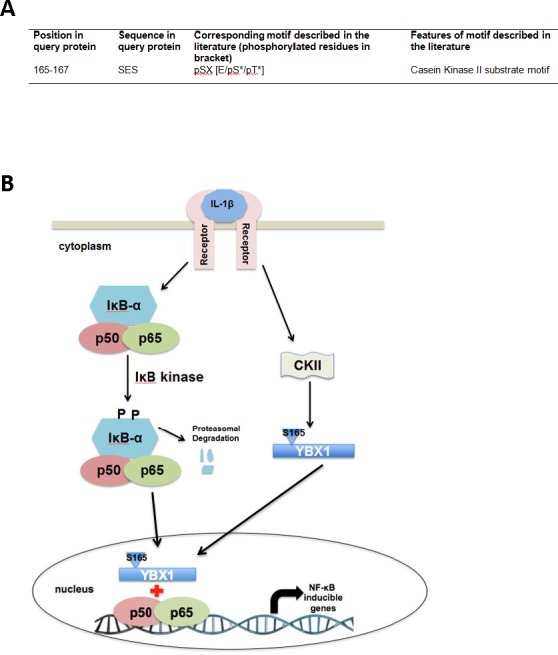
A. Casein kinase II is predicted to be the kinase that is responsible for the phosphorylation of S165 of YBX1. B. Model, hypothesizing that in the presence of external stimuli like IL-1β, the IκB kinase phosphorylates IκBα. This leads to the degradation of IκB. The free P65/P50 heterodimer then migrate to the nucleus to bind to κB-binding sites on the promoters of specific genes, leading to their activation. On the other hand, IL-1β has also been shown to stimulate YBX1 activity. We hypothesize that stimulation with IL-1β activates CKII, which then promotes phosphorylation of YBX1 on S165 which then leads to increased activation of NF-κB, possibly by increasing the DNA binding ability of NF-κB, and enhancing the expression of NF-κB-inducible genes, thus promoting cancer progression.
