Abstract
Synthetic and biological materials are commonly used for pelvic floor reconstruction. In this study, host tissue response and biomechanical properties of mesh fabricated from co-electrospun poly(l-lactide-co-caprolactone) (PLCL) and fibrinogen (Fg) were compared with those of polypropylene mesh (PPM) in a canine abdominal defect model. Macroscopic, microscopic, histological, and biomechanical evaluations were performed over a 24-week period. The results showed that PLCL/Fg mesh had similar host tissue responses but better initial vascularization and graft site tissue organization than PPM. The efficacy of the PLCL/Fg mesh was further examined in human pelvic floor reconstruction. Operation time, intraoperative blood loss, and pelvic organ prolapse quantification during 6-month follow-up were compared for patients receiving PLCL/Fg mesh versus PPM. According to the pelvic organ prolapse quantification scores, the anterior vaginal wall 3 cm proximal to the hymen point (Aa point), most distal edge of the cervix or vaginal cuff scar point (C point), and posterior fornix point (D point) showed significant improvement (P<0.01) at 1, 3, and 6 months for both groups compared with preoperatively. At 6 months, improvements at the Aa point in the PLCL/Fg group were significantly more (P<0.005) than the PPM group, indicating that, while both materials improve the patient symptoms, PLCL/Fg mesh resulted in more obvious improvement.
Keywords: anterior pelvic floor reconstruction, polypropylene mesh, poly(l-lactide-co-caprolactone), fibrinogen, pelvic organ prolapse quantification
Introduction
Pelvic organ prolapse (POP) occurs when the supporting muscles and connective tissue of the pelvic floor are damaged as a result of childbirth, injury, and aging, resulting in the pelvic organs dropping from their normal position. It is estimated that ~50% of parous women will experience some degree of POP; 20% of these require surgical treatment.1 Most common is the anterior vaginal wall prolapse, which occurs 2–3 times more frequently than the posterior vaginal prolapse (vault prolapse).2 A common method of treating POP is pelvic floor reconstruction, which is intended to restore normal pelvic anatomical structure and relieve symptoms while preventing recurrence rate. Implantation of mesh materials for pelvic floor reconstruction has benefits over traditional repairs. Depending on their nature, mesh materials can be categorized into two types: synthetic materials and biological materials. Synthetic materials, such as polypropylene mesh (PPM), have been widely used in clinical applications; however, the major drawback of tissue erosion remains a problem. Compared with the permanent mesh, biological materials have the advantage of biodegradation and better biocompatibility, and are an alternative to PPM. Biological materials derived from autologous fascia (fascia lata and rectus fascia), porcine dermis, small intestine submucosa, and bovine pericardium have been used in POP repair.3 However, the limitations of biologically derived mesh include the size of materials, potentially viral or prion transmission, and relatively low mechanical strength.4 The mechanical strength of biological meshes can be enhanced with cross-linking processes, but many animal studies have shown that cross-linking has a negative effect on the degradation profile and host response to the materials.5–7 Currently, there is no consensus on the optimal material for POP repair.
Electrospinning techniques for scaffold fabrication have resulted in meshes with promising advantages such as large surface area to volume ratio, high porosity, and a nanofibrous structure resembling the extracellular matrix (ECM) in the body.8,9 Nanofibrous poly(l-lactide-co-caprolactone) (PLCL), a copolymer of lactide and caprolactone, is of particular interests for soft tissue engineering because of its elasticity, flexibility, biocompatibility, and controlled degradation properties.10,11 In the previous study, we prepared the meshes by co-electrospinning of PLCL with fibrinogen (Fg) and demonstrated good biocompatibility and bioactivity.12
It is necessary to use animal models to understand host tissue responses to the materials and their mechanical properties after implantation. For pelvic floor reconstruction, the nonhuman primates are considered as the best model because of their similarities to human anatomy and the function of muscular and connective tissues of the pelvic floor.13 However, the nonhuman primate models are limited because of low sample size and ethical questions concerning their use. Therefore, many in vivo studies of POP meshes have been performed with abdominal wall models of other species that offer the advantages of easy access for implantation and large area for sample retrieval for multiple tests.13–16
In this study, the properties of PLCL/Fg mesh and PPM were compared in a canine abdominal defect model. In addition, the efficacy of PLCL/Fg in human anterior pelvic floor reconstruction was evaluated using the POP quantification (POP-Q) system. Here, we present the data obtained from both animals and humans to provide detailed information about host responses and biomechanical properties of PLCL/Fg and PPM from in vivo studies and efficacy assessment in clinical trials.
Materials and methods
Preclinical study
Experimental animals
Thirty beagle dogs (15 males and 15 females), each weighing 15–20 kg, were purchased from Shanghai Jiao Tong University School of Agriculture and Biology (Shanghai, People’s Republic of China). The dogs were randomly numbered and divided into five groups of six dogs each. All animal study protocols were approved by the Institutional Review Committee of Shanghai Jiao Tong University School of Materials Science and Engineering. The dogs were housed in accordance with the national guidelines for animal welfare. Six animals from each group were sacrificed at the following time: 1, 2, 4, 12, and 24 weeks.
Implantation materials
The PPMs were purchased from C. R. Bard, Inc. (Atlanta, GA, USA). The nanofibrous PLCL/Fg mesh was fabricated, as previously described, by P&P Biotech Co. Ltd. (Shanghai, People’s Republic of China).12
Surgical procedures
Each dog was anesthetized with an intra-abdominal injection of pentobarbital sodium (14–20 mg/kg). The hairs on the ventral abdomen were shaved and the skin was disinfected with a povidone iodine solution. The surgical sites were located ~2.5 cm lateral to the linea alba bilaterally. After making a 7 cm skin incision, full-thickness defects 6 cm long ×4 cm wide were created in the muscle of the lateral abdominal wall. The peritoneum was kept intact. The left and right defects were randomly assigned to receive PPM or PLCL/Fg mesh. A 7×5 cm mesh was placed onlay on the defects and each side of the mesh had a 1 cm overlap with the muscle underneath. The mesh was then fixed with a 4/0 multifilament running sutures. The overlying subcutaneous tissue and skin were closed in a routine fashion. The animals were given gentamicin (40,000 U) for 3 days postoperatively.
Macroscopic examination
At the time of sacrifice, animal were examined by gross inspection for evidence of hernia, seroma, and implant infection before sampling. For sampling, the meshes together with at least 2 cm of surrounding tissue were excised and rinsed with saline solution. Sample thickness was measured by taking five random measurements with digital calipers. Changes in thickness were defined as the mean value measured at the time of sacrifice minus the original mesh thickness before implantation.17 The hardness of the explanted samples were recorded as soft, firm, or hard due to mineralization or cartilaginous calcification, and given a score of 1 (soft) to 5 (hard). The normal abdominal wall tissues were used as a reference with a score of 3. All the samples were evaluated in a blinded fashion to ensure the consistency.18
Histological staining
Retrieved samples with 1 cm surrounding tissues were trimmed and fixed in neutral buffered formaldehyde for 48 hours and then embedded in paraffin. Sections of 3 μm thickness slide were cut and stained with hematoxylin and eosin and Masson trichrome. Microscopic evidence of polymorphonuclear cells (PMNs), mononuclear cells (MNs), organization of collagen deposition, and vascularity within the defect were assessed using the scoring system described by Badylak et al18 (Table 1).
Table 1.
Scoring criteria for microscopic evaluation
| Category | Score
|
|||
|---|---|---|---|---|
| 0 | 1 | 2 | 3 | |
| Polymorphonuclear cells | 0 | 1–5 | 6–10 | >10 |
| Mononuclear cells | 0 | 1–5 | 6–10 | >10 |
| Collagen organization | Disorganized | Slightly organized | Moderately organized | Well organized |
| Vascularity | 0 | 1–3 | 4–10 | >10 |
Mechanical testing
Ball burst strength of the materials was assessed using a ball burst strength tester (SANSCM, Shanghai, People’s Republic of China) with a customized fixture (two circular stainless steel rings with an aperture of 44.5 mm for sample fixation). The test specimen was clamped in the fixture with the mid-portion of the sample (ie, explanted mesh or native tissue) centered directly over the circular aperture. A stainless steel ball with 25 mm diameter was applied in compression at a rate of 300 mm/min until the samples were burst. The ultimate burst strength was presented as N/mm with respect to the thickness of the tested samples. The explanted samples for mechanical testing were placed in 0.9% saline solution and tested within 4 hours of collection.19
Clinical trials
Between February and December 2013, 38 patients with POP (mainly anterior vaginal wall prolapse) requiring anterior pelvic compartment slingplasty and posterior vaginal wall repair were enrolled at the Department of Obstetrics and Gynecology, Shanghai Tongji Hospital, Tongji University, Shanghai, People’s Republic of China. Meshes were used during the anterior pelvic compartment slingplasty and the data from the surgery and follow-up visits were retrospectively analyzed. The enrolled patients were allowed independent choice of mesh type and were divided into two groups: the experimental group with PLCL/Fg mesh (18 patients) and the control group with Bard PPM (20 patients). Clinical trial protocols were approved by the Ethics Committee of Shanghai Tongji Hospital, affiliated with Tongji University.
Preoperative POP-Q evaluation
POP-Q evaluation of the patients in the experimental group before surgery revealed the followings: uterine (vault) prolapse at stage IV, two cases; stage III, five cases; stage II, eight cases; and stage I, one case. Anterior vaginal wall prolapse at stage IV, two cases; stage III, eight cases; and stage II, nine cases. Posterior vaginal wall prolapse at stage IV, one case; stage III, one case; stage II, five cases; and stage I, two cases. POP-Q evaluation of the patients in the control group before surgery revealed the following: uterine (vault) prolapse at stage III, seven cases; stage II, five cases; and stage I, four cases. Anterior vaginal wall prolapse at stage III, ten cases and stage II, nine cases. Posterior vaginal wall prolapse at stage III, one case; stage II, five cases; and stage I, four cases.
Mesh materials
Surgical instruments for pelvic floor reconstruction, including trocar, trocar cannulas, and fixation cannulas core were provided by Johnson and Johnson. The PLCL/Fg mesh was provided by P&P Biotech Co. Ltd.. PPM was purchased from Bard (Avaulta Solo Synthetic Support System, C. R. Bard, Inc.).
Surgical procedures
Surgeries were conducted by the same experienced gynecological surgeon. Anterior pelvic compartment slingplasty with or without posterior vaginal wall repair were performed in both groups. The procedures can be described as follows: after successful general anesthesia, the patients were placed in the lithotomy position and catheterized. Physiological saline was infiltrated into the submucosa of the anterior vaginal wall. After a 4 cm vertical incision was made in the anterior vaginal wall submucosa, the bilateral vesicovaginal interspace was separated. The bladder was pushed up during the separation and the separation was extended until it reached the descending pubic ramus. A midline incision was made in the anterior vaginal wall. The dissection extended laterally under the base of the bladder, penetrating deep into the retropubic space. Four millimeter skin incisions were made at 4 cm lateral (distal pass) and 2 cm below (proximal pass) on both sides of the urethral opening. The needle perforated the obturator externus muscle and then the inferior middle point of the obturator membrane and was advanced to the approximate depth of the ischial spine. Once the needle tip penetrated the obturator internus muscle, it was advanced ~1 cm into the dissected space. The guide wire loop was pushed out and then the mesh tip was inserted into the loop. The arms of the mesh were brought out by rotating the needle back out. The needle tip was passed through superior groin incision and directed to the level of the bladder neck. The needle was guided through the obturator internus as before. The introducer needle was retracted to draw the mesh arm out through the superior groin incision. The arms of mesh were drawn into the desired position to ensure that the mesh was tension-free. The mesh was sutured to the bladder–cervical fascia. The anterior colpotomy was closed with a running 2/0 suture from the urethral meatus to the vaginal apex. The time of operation and blood loss volume were recorded for all the patients.
Postoperative follow-up visits
Follow-up visits occurred at 1, 3, and 6 months after surgery. Wound recovery, POP-Q, mesh rejection, erosion, and exposure were assessed during the follow-up visits.
Statistics
Unpaired Student’s t-tests were used to compare the groups; Kruskal–Wallis tests were used to compare the differences between PPM and PLCL/Fg samples on macroscopic and microscopic examinations and the mechanical properties at different time points. Data are presented as mean ± standard deviation. Value of P<0.005 indicate statistical significance.
Results
Macroscopic examination
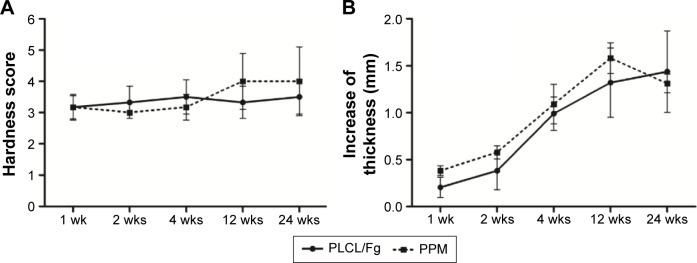
The hardness score of the PLCL/Fg mesh and PPM remained between 3 and 4, although the PPM showed a trend of slight increase in hardness as the study progressed to 24 weeks (Figure 1A). Both PLCL/Fg and PPM showed a relatively slow increase of thickness from 1 to 2 weeks, then a faster increase from 2 to 12 weeks (Figure 1B); thickness at 12 and 24 weeks was similar. At 4 weeks’ time, there was a significant increase in PLCL/Fg and PPM thickness at the implantation site, compared with before implantation (0.60±0.045 for PLCL/Fg and 0.56±0.019 mm for PPM, P<0.05).
Figure 1.
Hardness and thickness of PLCL/Fg and PPM at the implant site during 24 weeks of study. (A) Hardness of the PLCL/Fg and PPM after implantation. (B) Increase in thickness of PLCL/Fg and PPM after implantation.
Abbreviations: Fg, fibrinogen; PLCL, poly(l-lactide-co-caprolactone); PPM, polypropylene mesh; wks, weeks.
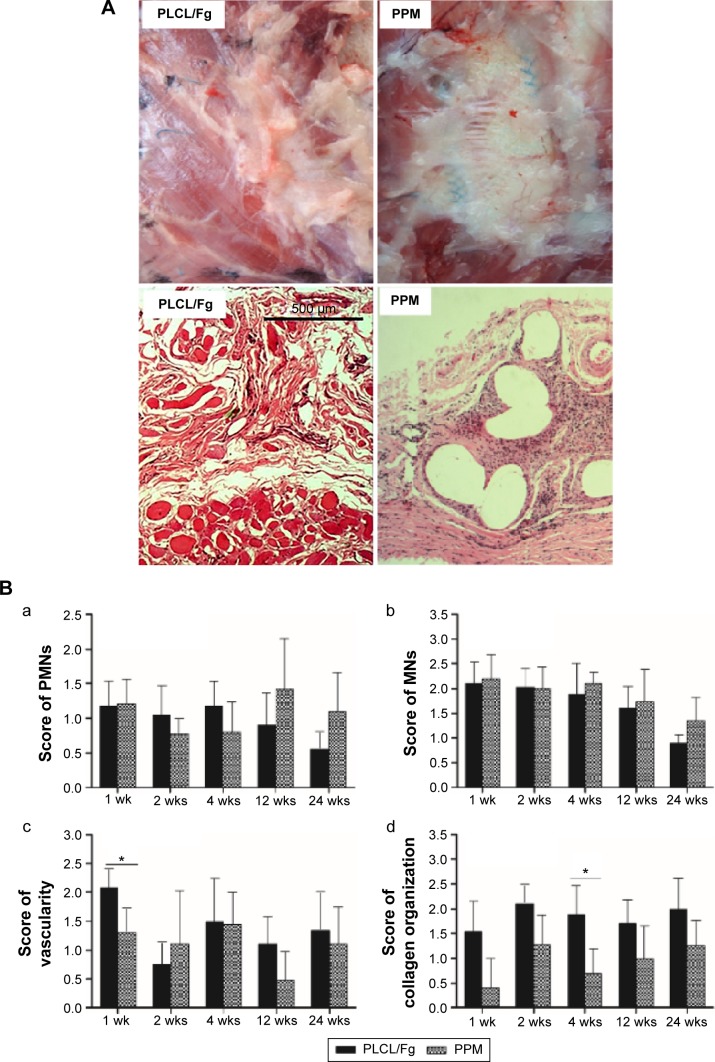
On the gross observation at 24 weeks (Figure 2A), the defect area repaired with PLCL/Fg mesh was filled with tissue similar to adjacent abdominal muscles. The defect area repaired with PPM showed a white glistening surface with an encapsulated implant.
Figure 2.
(A) Representative cross-sections of PLCL/Fg and PPM samples stained with hematoxylin and eosin at 24 weeks time points (scale bar =500 μm, ×40). (B) Histological analysis of PLCL/Fg and PPM after implantation. (a) Average score for the number of polymorphonuclear cells. (b) Average score for the number of mononuclear cells. (c) Amount of vascularity. (d) Degree of collagen organization. *P<0.05.
Abbreviations: Fg, fibrinogen; MNs, mononuclear cells; PLCL, poly(l-lactide-co-caprolactone); PMNs, polymorphonuclear cells; PPM, polypropylene mesh; wks, weeks.
Microscopic observations after implantation
Figure 2A shows muscular tissue mixed with collagen fibrous tissues 24 weeks after defect repair with PLCL/Fg. The PPM shows encapsulation by fibrous tissue around the filaments with some inflammatory cells.
The average scores for PMNs, MNs, vascularity, and collagen fiber organization are presented in Figure 2B. Host tissue responses at the implantation site were similar for PLCL/Fg and PPM. Cellular infiltration of a mixture of PMNs and MNs appeared 1 week after implantation and then gradually decreased over time (Figure 2B a and b). The PLCL/Fg mesh showed significantly higher initial in-growth of new blood vessels into the implantation site (P<0.05) than the PPM at 1 week. The vascularity scores for the PLCL/Fg and PPM after 2 weeks were similar (P>0.05) and remained steady over the remaining weeks.
Mechanical properties and histological observation after implantation
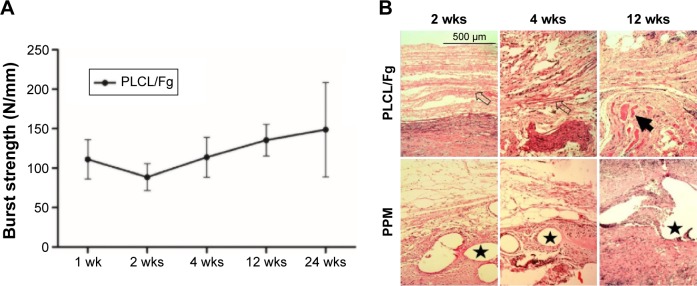
The mechanical properties of PPM before and after implantation were well documented and therefore were not studied here.20–22 The mechanical properties of PLCL/Fg after implantation were evaluated using ball burst strength tests (Figure 3A). The burst strength of PLCL/Fg prior to implantation was 380.6±24.7 N/mm. The burst strength for the native abdominal wall tissue was ~75 N/mm (removed at the time of surgery). In the first 2 weeks after implantation, there was a rapid decrease in strength of the PLCL/Fg to a value of 88.7±17.2 N/mm; this average value was still above that of the native abdominal wall. After 2 weeks, the burst strength of the implanted PLCL/Fg steadily increased, reaching 148.7±59.9 N/mm at 24 weeks. In addition, the molecular weight of the PLCL/Fg after implantation was measured by gel permeation chromatography. The samples were collected at 1, 2, and 4 weeks when the material could still be distinguished from the surrounding tissue. The molecular weight of the PLCL/Fg at 0, 1, 2, and 4 weeks were 137,200, 136,300, 130,600, and 118,200 Da, respectively. Hematoxylin and eosin staining of the explanted tissues were conducted at 2, 4, and 12 weeks. As shown in Figure 3B, the aligned PLCL/Fg mesh was clearly visible with pronounced inflammatory cell infiltration at 2 weeks. The PLCL/Fg structure became less visible and more tissue had infiltrated into the mesh at 4 weeks. At 12 weeks, the PLCL/Fg appeared to have reduced inflammation and there were visible muscle tissues mixed with fibrous collagen tissue. The filaments in the PPM group were clearly visible throughout the course of the study. Encapsulation of fibrous tissue around the filaments and chronic inflammation were generally observed in the PPM group.
Figure 3.
(A) Ball burst strength of PLCL/Fg after implantation for 24 weeks. (B) Representative cross-sections of PLCL/Fg and PPM samples stained with hematoxylin and eosin at 2, 4, and 12 weeks (scale bar =500 μm, ×40). The mesh structure of PLCL/Fg is indicated with hollow arrowheads, the regenerated muscle tissues are indicated with solid arrowhead, and the PPM filaments are indicated with stars.
Abbreviations: Fg, fibrinogen; PLCL, poly(l-lactide-co-caprolactone); PPM, polypropylene mesh; wks, weeks.
Clinical study of PLCL/Fg meshes: intraoperative situation
The intraoperative time for the procedures using PLCL/Fg meshes was slightly longer than those for PPMs. Blood loss in the PLCL/Fg surgeries was greater than in PPM surgeries. However, these differences were not statistically significant (P>0.05), as shown in Table 2.
Table 2.
Comparison of operation time and blood loss for PLCL/Fg and PPM groups
| Patients | Operation time (minutes) | Blood loss (mL) | |
|---|---|---|---|
| PLCL/Fg | 19 | 50.73±15.26 | 65.26±41.34 |
| PPM | 19 | 50.21±26.16 | 50.99±47.00 |
| t-value | 0.037 | −0.25 | |
| P-value | 0.103 | 0.8 |
Abbreviations: Fg, fibrinogen; PLCL, poly(l-lactide-co-caprolactone); PPM, polypropylene mesh.
Follow-up visits
Postoperative follow-up visits
The date of the last 6 month follow-up visits for patients was in March 2014 with follow-up rate of 92% (35/38). Two patients who received PLCL/Fg mesh and one who received PPM were lost to follow-up. In the PPM group, one patient had erosion of the mesh at month 4 and the mesh was removed after re-examination.
POP-Q system
POP-Q was conducted at 1, 3, and 6 months after surgery. The anterior and apical points were measured and compared between the PLCL/Fg and PPM groups. The value of POP-Q at the anterior vaginal wall 3 cm proximal to the hymen (Aa point), the most distal edge of the cervix or vaginal cuff scar (C point), and the posterior fornix (D point) were significantly improved in both groups after 1, 3, and 6 months compared with before surgery (P<0.01) (Table 3).
Table 3.
Comparison of preoperative and postoperative POP-Q of women undergoing repair with PLCL/Fg versus PPM
| PLCL/Fg group
|
PPM group
|
|||||
|---|---|---|---|---|---|---|
| Aa | C | D | Aa | C | D | |
| Preoperation | 1.20±1.84 | 0.52±3.41 | −2.32±3.47 | 1.52±0.97 | 1.27±3.27 | −3.05±2.24 |
| 1 month postoperation | −3 | −5.82±1.05 | −7.02±0.96 | −3 | −4.94±1.12 | −5.63±2.77 |
| t-value | 9.106 | 8.690 | 6.767 | 19.078 | 7.500 | 4.397 |
| P-value | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| Preoperation | 1.20±1.84 | 0.52±3.41 | −2.32±3.47 | 1.52±0.97 | 1.27±3.27 | −3.05±2.24 |
| 3 months postoperation | −1.73±0.68 | −4.38±1.39 | −6±1.17 | −3 | −4.88±1.09 | −5.47±2.66 |
| t-value | 5.951 | 5.504 | 5.435 | 19.078 | 7.590 | 4.246 |
| P-value | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.001 |
| Preoperation | 1.20±1.84 | 0.52±3.41 | −2.32±3.47 | 1.52±0.97 | 1.27±3.27 | −3.05±2.24 |
| 6 months postoperation | −0.50±1.08 | −3.79±1.52 | −5.73±1.01 | −3 | −4.72±1.08 | −5.38±2.62 |
| t-value | 3.088 | 4.712 | 4.905 | 19.078 | 7.911 | 4.255 |
| P-value | 0.007 | 0.000 | 0.000 | 0.000 | 0.000 | 0.001 |
Abbreviations: Aa, anterior vaginal wall 3 cm proximal to the hymen point; C, most distal edge of the cervix or vaginal cuff scar point; D, posterior fornix point; Fg, fibrinogen; PLCL, poly(l-lactide-co-caprolactone); POP-Q, pelvic organ prolapse quantification; PPM, polypropylene mesh.
At 6 months, the improvement at the Aa point in the PLCL/Fg group was significantly better than the PPM group (P<0.05); however, there was no significant difference between groups in improvement at the C and D points (P>0.05) (Table 4).
Table 4.
Comparison of 6-month postoperative POP-Q of women undergoing PLCL/Fg and PPM repair
| Aa | C | D | |
|---|---|---|---|
| PLCL/Fg group | −0.50±1.08 | −3.79±1.52 | −5.73±1.01 |
| PPM group | −3 | −4.72±1.08 | −5.38±2.62 |
| t-value | 4.693 | 1.415 | −1.123 |
| P-value | 0.017 | 0.445 | 0.205 |
Abbreviations: Aa, anterior vaginal wall 3 cm proximal to the hymen point; C, most distal edge of the cervix or vaginal cuff scar point; D, posterior fornix point; Fg, fibrinogen; PLCL, poly(l-lactide-co-caprolactone); PPM, polypropylene mesh; POP-Q, pelvic organ prolapse quantification.
Discussion
The use of mesh in pelvic floor reconstruction treatment not only allows reconstruction of anatomical structure and improves pelvic floor function, but also effectively reduces the recurrence rate compared with the conventional treatment. Currently, the PPM is one of the most used meshes in clinics for pelvic floor reconstruction.4 Falagas et al23 reported an infection rate of 0%–8% and an erosion rate of 0%–33% with PPM, problems which have not been overcome.23 Because biological meshes have the advantage of being biocompatible and tissue inductive, more and more surgeons and researchers have become interested in the biological meshes for pelvic floor reconstruction.24,25 However, recurrence rates as high as 39% have been reported for biological mesh derived from fascia lata because of mechanical strength issues and rate of biodegradation.26
We have developed biodegradable meshes containing PLCL and Fg via electrospinning technology. After implantation at the abdominal defect site, the initial host response to the PLCL/Fg and PPM were similar, characterized by infiltration of PMNs and MNs and followed by deposition of host ECM. However, significant differences in host tissue responses were seen, including greater initial vascularization and better organization of connective tissue at later stage at the implantation site with PLCL/Fg. The positive tissue responses to PLCL/Fg seen in this study could be related to the nanostructure and surface property of the mesh. Nanofibers of the PLCL/Fg mesh are similar in the structure and organization to the collagen fibers of native ECM; this structure facilitates cell adhesion, the processes of cellular matrix deposit, and would healing.27 In our previous in vitro study, we also found that the PLCL/Fg scaffold promoted cell attachment on the surface due to the nanostructure and its hydrophilicity.12 Our findings were consistent with those of other authors, that nanoscale fibers improve cell proliferation and elicit superior metabolic and matrix forming activities.28,29
The biomechanical properties of PLCL/Fg after implantation showed a trend of rapid decrease in strength at 2 weeks followed by steady increase in mechanical strength. This trend can be attributed to PLCL/Fg degradation (demonstrated by a decrease in its molecular weight), cell infiltration, neovascularization, and deposition of host matrix. After the degradation and new tissue formation reached a balance, the mechanical strength was further enhanced by the combination of neotissue regeneration and remodeling. The change in the biomechanical properties of PLCL/Fg after implantation were consistent with the findings for small intestinal submucosa; the mesh had an ~45% decrease in the burst load in the first 10 days after implantation, with a subsequent increase in the strength comparing with the starting material after 1 month.19 In our study, the lowest ball burst strength was recorded ~2 weeks after surgery; however, the biomechanical strength of the implantation site remained above the native tissue at all the testing points.
In this study, we used POP-Q to compare the efficacy of the PLCL/Fg and PPM in the treatment of pelvic floor reconstruction at 1, 3, and 6 months after operation. The results of Aa, C, and D points measurements were significantly improved (P<0.05) after surgery for patients receiving both PLCL/Fg and PPM. This finding indicates that the application of both types of mesh in the anterior pelvic sling operation improved the patients’ POP symptom. In the PPM group, one patient (5%) had mesh erosion and asked to have it removed after 4 months. The PLCL/Fg group had no occurrence of mesh erosion, POP reoccurrence, foreign body sensation, or dyspareunia during the same period of study. At 6 month follow-up, the POP-Q value at the Aa point for patients who had received PLCL/Fg was significantly improved (P<0.05) compared with the PPM; however, the values at the C and D points were not significantly different (P>0.05). This finding reveals that the PLCL/Fg meshes have a better effect on improving symptoms in patients with anterior vaginal prolapse than the PPM. However, the absorbable time for the studied biological mesh is ~4 months; further investigation will be needed to study the long-term efficacy.
Conclusion
In conclusion, this in vivo study showed that the PLCL/Fg resulted in earlier vascularization, better collagen fiber organization and muscle regeneration compared with the PPM. Application of either PLCL/Fg or PPM in anterior pelvic floor reconstruction improves the symptoms. Surgeons should inform patients about their mesh options prior to surgery. In short-term efficacy, PLCL/Fg mesh had no erosion and had a better effect on improving patient anterior vaginal prolapse than PPM. However, because of the small number of patients and short follow-up period in this study, the long-term efficacy and complications need further investigation.
Footnotes
Disclosure
No financial support or benefits have been received by XJW, YRW, XWT, and HFL from any commercial source that is related directly or indirectly to the scientific work reported on in the paper, except as described as follows. HBH is one of the founders and stockholders of P&P Biotech Co., Ltd; CCZ, MY, ZL, WHH, FW, and HHZ are employed by Shanghai Pine & Power Biotech Co., Ltd. (Shanghai, People’s Republic of China) who provided support for research activities, writing, and editing of the paper, but otherwise played no role in the study design or implementation. The authors report no other conflicts of interest in this work.
References
- 1.Phillips CH, Anthony F, Benyon C, Monga AK. Collagen metabolism in the uterosacral ligaments and vaginal skin of women with uterine prolapse. BJOG. 2006;113(1):39–46. doi: 10.1111/j.1471-0528.2005.00773.x. [DOI] [PubMed] [Google Scholar]
- 2.Mettu JR, Colaco M, Badlani GH. Evidence-based outcomes for mesh-based surgery for pelvic organ prolapse. Curr Opin Urol. 2014;24(4):370–374. doi: 10.1097/MOU.0000000000000063. [DOI] [PubMed] [Google Scholar]
- 3.Birch C, Fynes MM. The role of synthetic and biological prostheses in reconstructive pelvic floor surgery. Curr Opin Obstet Gynecol. 2002;14(5):527–535. doi: 10.1097/00001703-200210000-00015. [DOI] [PubMed] [Google Scholar]
- 4.Gigliobianco G, Roman Regueros S, Osman NI, et al. Biomaterials for pelvic floor reconstructive surgery: how can we do better? Biomed Res Int. 2015;2015:968087. doi: 10.1155/2015/968087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Butler CE, Burns NK, Campbell KT, Mathur AB, Jaffari MV, Rios CN. Comparison of cross-linked and non-cross-linked porcine acellular dermal matrices for ventral hernia repair. J Am Coll Surg. 2010;211(3):368–376. doi: 10.1016/j.jamcollsurg.2010.04.024. [DOI] [PubMed] [Google Scholar]
- 6.Baumann DP, Butler CE. Bioprosthetic mesh in abdominal wall reconstruction. Semin Plast Surg. 2012;26(1):18–24. doi: 10.1055/s-0032-1302461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sandor M, Xu H, Connor J, et al. Host response to implanted porcine-derived biologic materials in a primate model of abdominal wall repair. Tissue Eng Part A. 2008;14(12):2021–2031. doi: 10.1089/ten.tea.2007.0317. [DOI] [PubMed] [Google Scholar]
- 8.Sill TJ, von Recum HA. Electrospinning: applications in drug delivery and tissue engineering. Biomaterials. 2008;29(13):1989–2006. doi: 10.1016/j.biomaterials.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 9.Ingavle GC, Leach JK. Advancements in electrospinning of polymeric nanofibrous scaffolds for tissue engineering. Tissue Eng Part B Rev. 2014;20(4):277–293. doi: 10.1089/ten.TEB.2013.0276. [DOI] [PubMed] [Google Scholar]
- 10.Xie J, Han Z, Naito M, et al. Articular cartilage tissue engineering based on a mechano-active scaffold made of poly(L-lactide-co-epsilon-caprolactone): in vivo performance in adult rabbits. J Biomed Mater Res B Appl Biomater. 2010;94(1):80–88. doi: 10.1002/jbm.b.31627. [DOI] [PubMed] [Google Scholar]
- 11.Xie J, Ihara M, Jung Y, et al. Mechano-active scaffold design based on microporous poly(L-lactide-co-epsilon-caprolactone) for articular cartilage tissue engineering: dependence of porosity on compression force-applied mechanical behaviors. Tissue Eng. 2006;12(3):449–458. doi: 10.1089/ten.2006.12.449. [DOI] [PubMed] [Google Scholar]
- 12.Fang Z, Fu W, Dong Z, et al. Preparation and biocompatibility of electrospun poly(l-lactide-co-ε-caprolactone)/fibrinogen blended nanofibrous scaffolds. Appl Surf Sci. 2011;257(9):4133–4138. [Google Scholar]
- 13.Couri BM, Lenis AT, Borazjani A, Paraiso MF, Damaser MS. Animal models of female pelvic organ prolapse: lessons learned. Expert Rev Obstet Gynecol. 2012;7(3):249–260. doi: 10.1586/eog.12.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klinge U, Klosterhalfen B, Birkenhauer V, Junge K, Conze J, Schumpelick V. Impact of polymer pore size on the interface scar formation in a rat model. J Surg Res. 2002;103(2):208–214. doi: 10.1006/jsre.2002.6358. [DOI] [PubMed] [Google Scholar]
- 15.Hilger WS, Walter A, Zobitz ME, Leslie KO, Magtibay P, Cornella J. Histological and biomechanical evaluation of implanted graft materials in a rabbit vaginal and abdominal model. Am J Obstet Gynecol. 2006;195(6):1826–1831. doi: 10.1016/j.ajog.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 16.Krause H, Goh J. Sheep and rabbit genital tracts and abdominal wall as an implantation model for the study of surgical mesh. J Obstet Gynaecol Res. 2009;35(2):219–224. doi: 10.1111/j.1447-0756.2008.00930.x. [DOI] [PubMed] [Google Scholar]
- 17.Liu Z, Tang R, Zhou Z, Song Z, Wang H, Gu Y. Comparison of two porcine-derived materials for repairing abdominal wall defects in rats. PLoS One. 2011;6(5):e20520. doi: 10.1371/journal.pone.0020520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Badylak S, Kokini K, Tullius B, Simmons-Byrd A, Morff R. Morphologic study of small intestinal submucosa as a body wall repair device. J Surg Res. 2002;103(2):190–202. doi: 10.1006/jsre.2001.6349. [DOI] [PubMed] [Google Scholar]
- 19.Badylak S, Kokini K, Tullius B, Whitson B. Strength over time of a resorbable bioscaffold for body wall repair in a dog model. J Surg Res. 2001;99(2):282–287. doi: 10.1006/jsre.2001.6176. [DOI] [PubMed] [Google Scholar]
- 20.Deeken CR, Abdo MS, Frisella MM, Matthews BD. Physicomechanical evaluation of absorbable and nonabsorbable barrier composite meshes for laparoscopic ventral hernia repair. Surg Endosc. 2011;25(5):1541–1552. doi: 10.1007/s00464-010-1432-0. [DOI] [PubMed] [Google Scholar]
- 21.Deeken CR, Abdo MS, Frisella MM, Matthews BD. Physicomechanical evaluation of polypropylene, polyester, and polytetrafluoroethylene meshes for inguinal hernia repair. J Am Coll Surg. 2011;212(1):68–79. doi: 10.1016/j.jamcollsurg.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 22.Obermiller JF, Hodde JP, McAlexander CS, Kokini K, Badylak SF. A comparison of suture retention strengths for three biomaterials. Med Sci Monit. 2004;10(1):PI1–PI5. [PubMed] [Google Scholar]
- 23.Falagas ME, Velakoulis S, Iavazzo C, Athanasiou S. Mesh-related infections after pelvic organ prolapse repair surgery. Eur J Obstet Gynecol Reprod Biol. 2007;134(2):147–156. doi: 10.1016/j.ejogrb.2007.02.024. [DOI] [PubMed] [Google Scholar]
- 24.Bellows C, Alder A, Helton W. Abdominal wall reconstruction using biological tissue grafts: present status and future opportunities. Expert Rev Med Devices. 2006;3(5):657–675. doi: 10.1586/17434440.3.5.657. [DOI] [PubMed] [Google Scholar]
- 25.Turner NJ, Badylak SF. Biologic scaffolds for musculotendinous tissue repair. Eur Cell Mater. 2013;25:130–143. doi: 10.22203/ecm.v025a09. [DOI] [PubMed] [Google Scholar]
- 26.Gandhi S, Goldberg RP, Kwon C, et al. A prospective randomized trial using solvent dehydrated fascia lata for the prevention of recurrent anterior vaginal wall prolapse. Am J Obstet Gynecol. 2005;192(5):1649–1654. doi: 10.1016/j.ajog.2005.02.061. [DOI] [PubMed] [Google Scholar]
- 27.Nisbet DR, Forsythe JS, Shen W, Finkelstein DI, Horne MK. Review paper: a review of the cellular response on electrospun nanofibers for tissue engineering. J Biomater Appl. 2009;24(1):7–29. doi: 10.1177/0885328208099086. [DOI] [PubMed] [Google Scholar]
- 28.Li WJ, Laurencin CT, Caterson EJ, Tuan RS, Ko FK. Electrospun nanofibrous structure: a novel scaffold for tissue engineering. J Biomed Mater Res. 2002;60(4):613–621. doi: 10.1002/jbm.10167. [DOI] [PubMed] [Google Scholar]
- 29.Zhang K, Wang H, Huang C, Su Y, Mo X, Ikada Y. Fabrication of silk fibroin blended P(LLA-CL) nanofibrous scaffolds for tissue engineering. J Biomed Mater Res A. 2010;93(3):984–993. doi: 10.1002/jbm.a.32504. [DOI] [PubMed] [Google Scholar]