Fig 5.
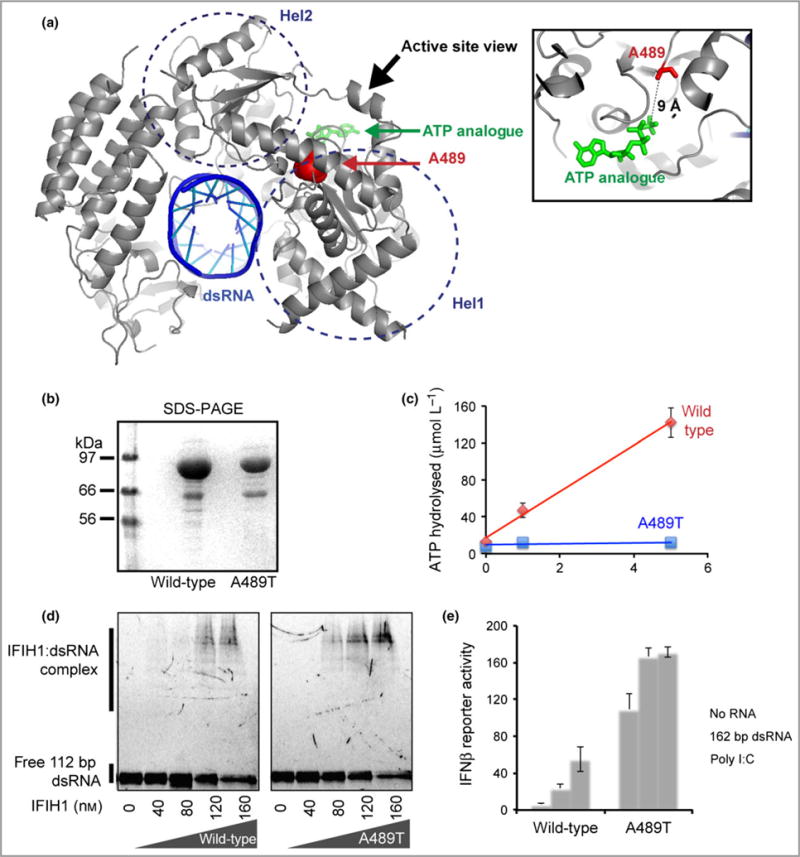
The p.Ala489Thr interferon-induced helicase C domain-containing protein 1 (IFIH1) mutant confers a gain of interferon signalling. (a) Mapping of the altered residue A489 (red sphere) onto the structure of IFIH1 Δ2CARD (grey) bound by double-stranded (ds)RNA (blue) and ATP analogue (green) (Protein Data Bank 4GL2). (b) Sodium dodecylsulfate polyacrylamide gel electrophoresis (SDS-PAGE) analysis (Coomassie stain) of the purified wild-type and mutant IFIH1. (c) ATP hydrolysis activity (mean ± SD, n = 3 biological replicates) of wild-type and mutant IFIH1. (d) Electrophoretic mobility shift assay of purified wild-type and mutant IFIH1 with 112-bp dsRNA. The gel image is representative of three independent experiments in the presence of ATP. (e) Interferon (IFN)-β reporter activity (mean ± SD, n = 3 biological replicates) of Flag-tagged wild-type and mutant IFIH1 with and without stimulation with 162-bp dsRNA or polyinosinic–polycytidylic acid (poly I:C) in HEK293T cells. The results are representative of three independent experiments.
