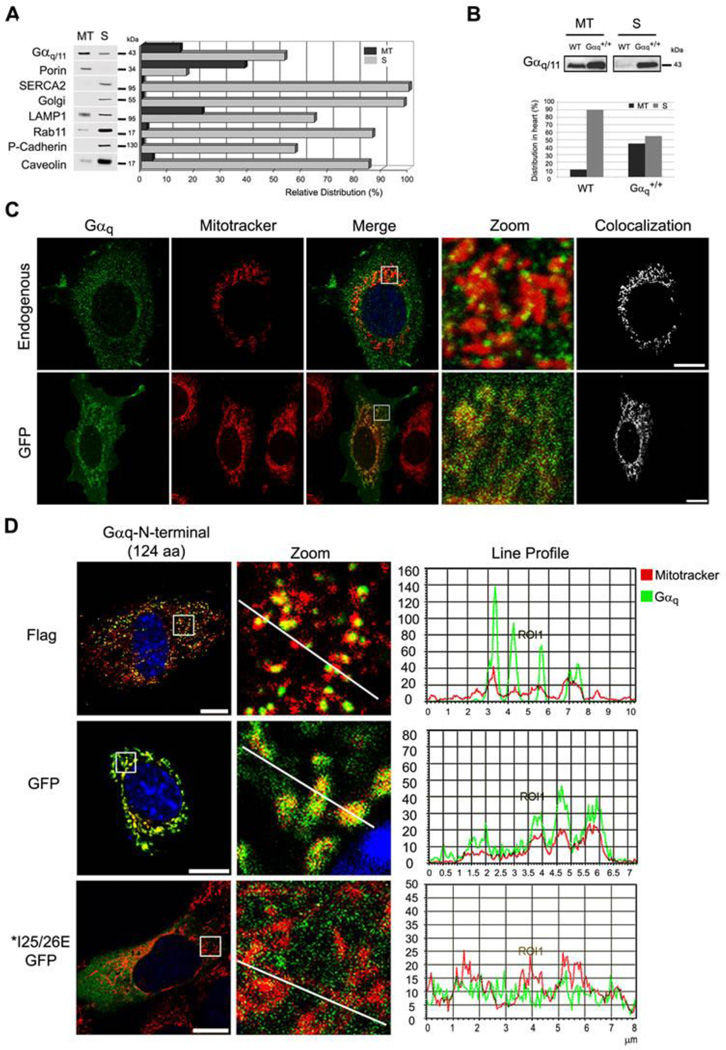
Fig. 1. Gαq and Gα11 are localized at the mitochondria.
(A) Distribution of endogenous Gαq/11 and organelle markers in the mitochondrial (MT) and supernatant (S) fractions obtained by Percoll gradient of NIH3T3 cells. The MT fraction was resuspended in 1/5 of initial volume, and equal volumes of MT and S fractions were loaded in the gel and immunoblotted with: anti-Gαq/11, Porin (mitochondria), SERCA2 (ER), Golgi, LAMP1 (lysosomes), Rab11 (ribosomes), P-cadherin (PM) and Caveolin-1 as a protein that binds to Gαq 35 and is present at the mitochondria 36. Quantification was performed by Multi-Gauge. (B) Gαq heart-specific transgenic mouse (Gαq+/+) show increased amount of Gαq at the mitochondria. The mitochondrial (MT) and supernatant fraction (S) were immunoblotted with Gαq/11 antibody. Quantification was performed by Alpha Ease FC. (C) Confocal micrographs of NIH3T3 cells (endogenous) immunostained with anti-Gαq/11 or expressing Gαq-GFP. Colocalization was performed by LAS AF. (D) Confocal micrographs of NIH3T3 cells incubated with mitotracker (red) and transfected with Gαq-N-terminus (1–124 aa) GFP and Flag, immunostained with anti-Flag and mounted with DAPI. MEF cells transiently expressing Gαq IE25/26AA mutants incubated with mitotracker (red), immunostained with anti-Gαq/11 and mounted with DAPI. Line profile was generated by LAS AF.