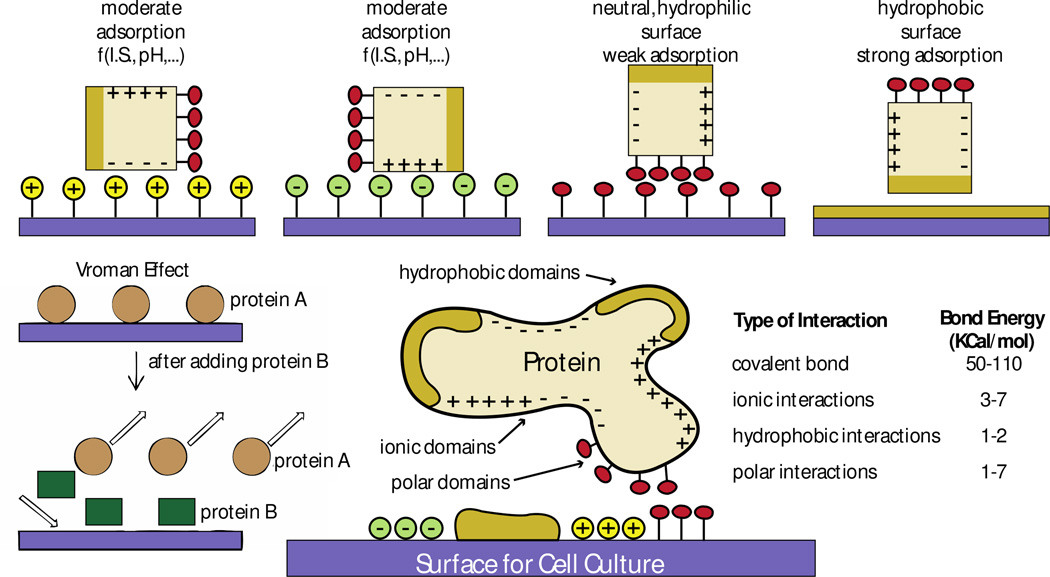
Figure 5. General mechanisms that govern protein-surface interactions based on surface chemistry.
Proteins interact with material surfaces and these interactions are regulated by both protein and surface properties. Interactions between ionic domains result in moderate protein adsorption, which is unstable because it relies on the ionic state (I.S) and the pH of the culture media. Interactions between hydrophilic polar domains are weak and also unstable. Interactions between hydrophobic domains lead to strong adsorption. Based on the mechanisms that regulate protein interaction (the Vroman effect), a protein B with higher affinity to a surface can replace a protein A of lower surface affinity previously adsorbed to the surface. Ideally, to maximize surface-protein interactions, surfaces should have different engineered regions with different chemical properties to match the properties of the protein of interest. Covalent interactions are the strongest among all types of interactions between proteins and surfaces. Figure adapted with permission from [86, 87]