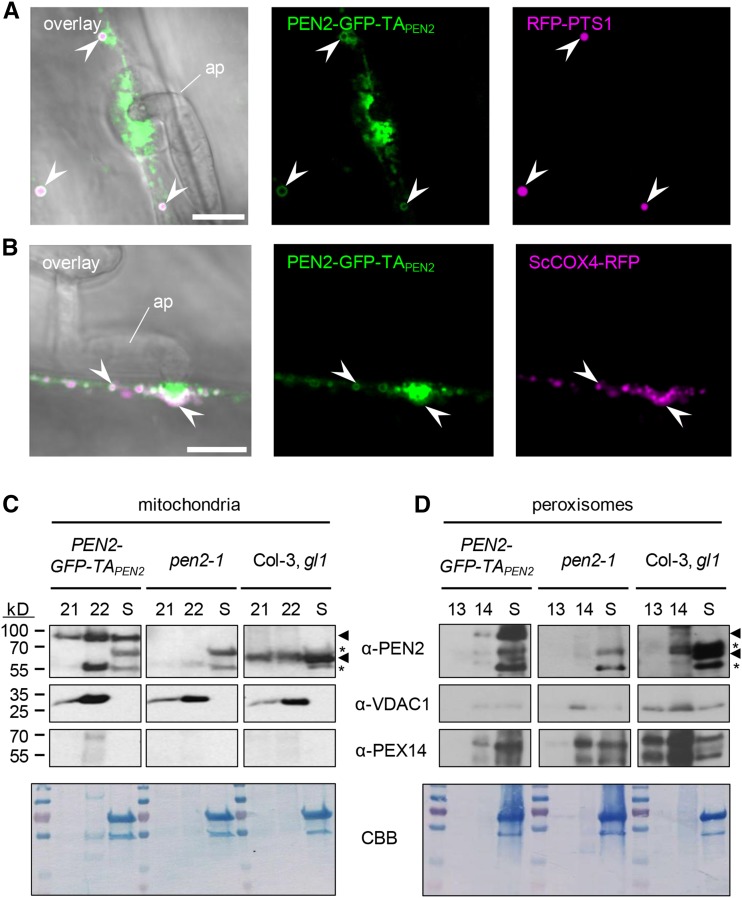
Figure 3.
PEN2 Is Localized to the Periphery of Peroxisomes and Mitochondria.
(A) CLSM image shows association of PEN2-GFP-TAPEN2 with the membrane of RFP-PTS1-marked peroxisomes in double transgenic leaf epidermal cells 20 hpi with Bgh. Arrowheads point to the localization of PEN2-GFP-TAPEN2 in the periphery of peroxisomes. Bar = 10 µm.
(B) CLSM image of double transgenic leaf epidermal cells expressing PEN2-GFP-TAPEN2 and mitochondrial marker ScCOX4-RFP confirms association of PEN2 with the membrane of mitochondria 22 hpi with Bgh. Arrowheads point to colocalization of mitochondria with PEN2-GFP-TAPEN2 aggregates and to association of PEN2-GFP-TAPEN2 with the periphery of this organelle. ap, appressorium. Bar = 10 µm.
(C) and (D) Immunoblot analyses of mitochondria-enriched (C) and peroxisome-enriched (D) fractions from Col-3, gl1 wild-type, pen2-1 knockout, and transgenic pen2-1 mutant plants expressing PEN2-GFP-TAPEN2. Following chloroplast sedimentation, the supernatant (S) was enriched for mitochondria or peroxisomes using Percoll (fractions 21 and 22 of 24 1.5-mL fractions) or sucrose (fractions 13 and 14 of 14 1-mL fractions) density gradients, respectively. Using a PEN2-specific antibody allowed the detection of endogenous PEN2 (∼65 kD, lower arrowhead) in wild-type plants and the detection of PEN2-GFP-TAPEN2 (∼95 kD, upper arrowhead) in transgenic pen2-1 mutant plants producing PEN2-GFP-TAPEN2 in both mitochondria (C) and peroxisome (D) fractions. Asterisks indicate unspecific signals detected by α-PEN2. Antibodies against the mitochondrial marker protein VDAC1 and the peroxisome-specific PEX14 were used to observe enrichment of the respective organelles. Note that due to small sample size and low protein concentration (∼30 µg), the same mitochondria (C) or peroxisome (D) fraction containing blot was used for probing with the indicated antibodies (involving stripping and reprobing). The Coomassie Brilliant Blue (CBB) staining shows loading on the respective membrane.