Abstract
Aims and Objectives:
To assess the diagnostic utility of contrast-enhanced 18F-fluorodeoxyglucose positron emission tomography-computed tomography (FDG PET-ceCT) in localization of tumors in patients with clinical diagnosis of tumor-induced osteomalacia (TIO), in correlation with histopathological results.
Materials and Methods:
Eight patients (five male and three female) aged 24–60 (mean 42) years with a clinical diagnosis of TIO were included in this prospective study. They underwent whole body (head to toe) FDG PET-ceCT following a standard protocol on Philips GEMINI TF PET-CT scanner. The FDG PET-ceCT results were correlated with postoperative histology findings and clinical follow-up.
Results:
All the patients had an abnormal PET-ceCT study. The sensitivity of PET-ceCT was 87.5%, and positive predictive value was 100%. The tumor was located in the craniofacial region in 6/8 patients and in bone in 2/8 patients. Hemangiopericytoma was the most common reported histology. All patients underwent surgery, following which they demonstrated clinical improvement. However, one patient with atypical findings on histology did not show any clinical improvement, hence, underwent 68Gallium-DOTANOC PET-ceCT scan for relocalization of the site of the tumor.
Conclusion:
The tumors causing TIO are small in size and usually located in obscure sites in the body. Hence, head to toe protocol should be followed for FDG PET-ceCT scans with the inclusion of upper limbs. Once the tumor is localized, regional magnetic resonance imaging can be performed for better characterization of soft tissue lesion. Imaging with FDG PET-ceCT plays an important role in detecting the site of the tumor and thereby facilitating timely management.
Keywords: Contrast-enhanced 18F-fluorodeoxyglucose positron emission tomography-computed tomography, fibroblast growth factor-23, hemangiopericytoma, phosphaturic mesenchymal tumors, tumor-induced osteomalacia
INTRODUCTION
Tumor-induced osteomalacia (TIO) is a rare paraneoplastic syndrome characterized by hyperphosphaturia, hypophosphatemia, decreased serum Vitamin D3 levels and osteomalacia. The underlying tumor in TIO may remain occult for months to a year and hence, the diagnosis is often established in fourth and fifth decades. Therefore, these tumors are described as “Strange tumors that occur in strange places.”[1] Overall these tumors are slow growing in nature, small in size and often are located in unusual sites, thus making its detection difficult. These characteristics make it imperative to perform whole body tomographic imaging with special emphasis on the appendicular skeleton. Various case reports and studies have emphasized the importance of whole body 18F-fluorodeoxyglucose positron emission tomography-computed tomography (FDG PET-CT) and recently 68Gallium-DOTANOC PET-CT (68Ga DOTANOC PET-CT) imaging in localization of site of the tumor.
This study describes findings in eight patients with clinical diagnoses of TIO, who underwent whole body contrast enhanced FDG PET-CT (FDG PET-ceCT) for localization of site of the tumor. FDG PET-ceCT was unable to locate the site of the tumor in a patient; hence, whole body contrast enhanced 68Ga DOTANOC PET-CT (68Ga DOTANOC PET-ceCT) imaging was performed for relocalization.
MATERIALS AND METHODS
Eight patients (five male and three female) aged 24–60 (mean 42) years with a clinical diagnosis of TIO were included in this prospective study. They were referred to Department of Nuclear Medicine and PET-CT for the whole body (head to toe) FDG PET-ceCT scan for detection of a primary tumor. The FDG PET-ceCT results were correlated with postoperative histology findings and clinical follow-up.
Scan protocol
The patients were fasting for 4–6 h prior to the scan. The blood glucose level <150 mg/dl was ensured. All the patients underwent whole body (head to toe + upper limbs) PET-ceCT with standard protocol on Philips GEMINI TF PET-CT scanner (Philips Medical Systems, Cleveland, Ohio, USA) with time of flight imaging and 64 slice computed tomography (CT) scanner. After 60 min of intravenous administration of 185–245 MBq of F-18-FDG emission data were acquired for 10–12-bed positions.
64-slice contrast-enhanced computed tomography (CECT) was used for attenuation correction, anatomical correlation, and diagnostic purpose. CT scans in all patients were performed craniocaudally using 120 kVp with a weight-based protocol for determining tube current (mA). A total volume of 100 ml of the intravenous contrast agent Omnipaque/Visipaque with an iodine concentration of 350 mg/ml was injected using a power injector at a flow rate of 2 ml/s, followed by 40 ml of saline at same flow rate.
RESULTS
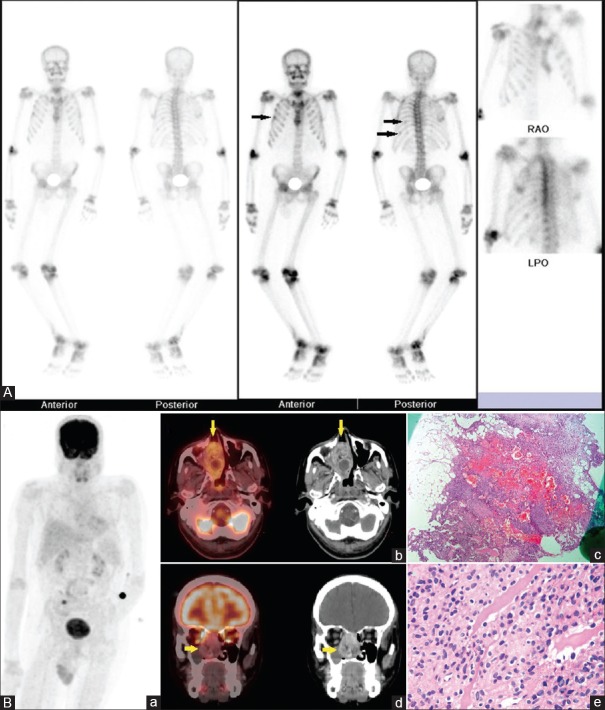
All eight cases had symptomatic osteomalacia with altered biochemical parameters. The patient characteristics are depicted in Table 1. There was delay in detection of tumors in almost all of cases since the time TIO was suspected. In seven cases, FDG PET-ceCT helped in confident localization of the tumor, thus making the sensitivity to be 87.5% and positive predictive value of 100%. FDG PET-ceCT and histopathology results were concordant in 7/8 patients. Hemangiopericytoma was the most common reported histology [Figure 1A and B]. The tumor was situated in bone in 2/8 patients and in the craniofacial region in 6/8 patients. These patients had their tumors removed with the alleviation of symptoms.
Table 1.
Summary of patient data
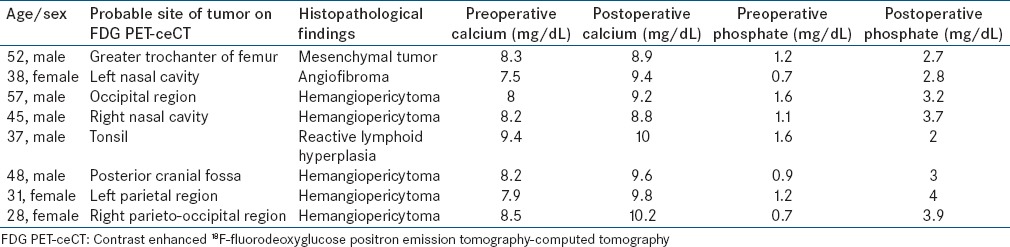
Figure 1.
(A) 45/M with polyarthritis for 7 years, progressive weakness of all 4 limbs and wasting of muscles for 5 years. Preoperative biochemical parameters: Serum calcium – 8.2 mg/dL, serum phosphorus – 1.1 mg/dL, serum Vitamin D3 – 14 ng/mL, serum parathyroid hormone – 171 pg/mL, serum alkaline phosphatase – 679 U/L, serum creatinine – 0.6 mg/dL. 99mTechnetium-methylene diphosphonate bone scintigraphy showing a diffuse increase in tracer concentration in axial and appendicular skeleton with microfractures in ribs (arrows) and faint visualization of kidneys suggestive of the metabolic bone disease. (B) Contrast-enhanced 18F-fluorodeoxyglucose positron emission tomography-computed tomography images. (a) Maximum intensity projection image showing physiological tracer distribution and increased fluorodeoxyglucose uptake in the right nasal region. (b) Transaxial and (d) coronal fused contrast enhanced positron emission tomography-computed tomography and corresponding contrast-enhanced computed tomography images showing increased fluorodeoxyglucose uptake (maximum standardized uptake value 5.2) in a soft tissue mass in right nasal cavity with whorl like enhancement on contrast. (c) Low power and (e) high power view photomicrographs showing spindle cell neoplasm with pericytomatous features – possibly sinonasal – type hemangiopericytoma. Postoperative serum calcium – 8.8 mg/dL, serum phosphorus – 3.7 mg/dL
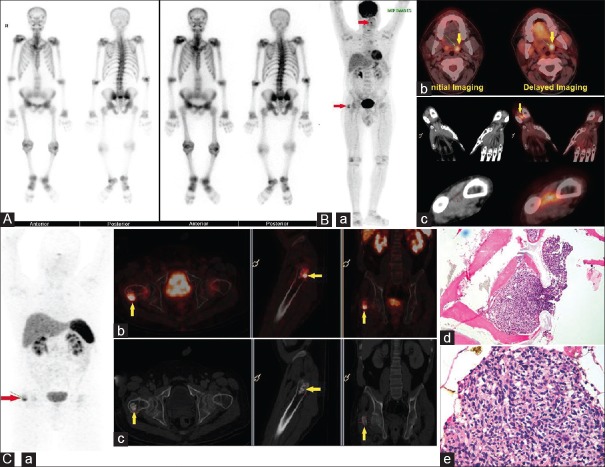
In one patient where FDG PET-ceCT was falsely positive, 68Ga-DOTANOC PET-ceCT scan was done for relocalization of site of the tumor [Figure 2A–C]. His FDG PET-ceCT scan showed a focus of metabolic activity with no corresponding CT abnormality in the region of left lingual tonsil and in the right distal forearm. Furthermore, FDG uptake was seen in a fibrous dysplasia like lesion in greater trochanter of the right femur. Regional magnetic resonance imaging (MRI) of head and neck also showed restricted diffusion in the region of left lingual tonsil. Screening MRI of the right hand was normal. The patient underwent tonsillectomy, and reactive lymphoid hyperplasia was documented in the histopathological examination. Bone curettage and grafting was done for lesion in greater trochanter of the right femur. There was no significant improvement in biochemical parameters and in symptoms of the patient after surgery. Hence, it was decided to do somatostatin receptor (SSTR) imaging for relocalization, as these tumors express the variable pattern of SSTRs. Ga DOTANOC PET-CT showed the focus of increased metabolic activity in the greater trochanter of right femur just adjacent to bone chips. He underwent bilateral total hip replacement. Histopathology from left total hip replacement specimen revealed atypical bland spindle cells. The patient showed gradual improvement in terms of symptoms and biochemical parameters.
Figure 2.
(A) 40/M with pain in bilateral hips for 2 years and difficulty in walking with insufficiency fracture in the neck of right femur with nonunion. Preoperative biochemical parameters: Serum calcium – 9.4 mg/dL, serum phosphorus – 1.6 mg/dL, serum Vitamin D3 – 10 ng/mL, serum parathyroid hormone – 43 pg/mL, serum fibroblast growth factor-23 – 3360 RU/mL, serum creatinine – 0.8 mg/dL. 99mTechnetium-methylene diphosphonate bone scintigraphy suggestive of the metabolic bone disease. (B) 18F-fluorodeoxyglucose positron emission contrast-enhanced computed tomography images (a) maximum intensity projection image showing focal fluorodeoxyglucose uptake in region of left lingual tonsil (maximum standardized uptake value 4.4) and in region of greater trochanter of right femur (maximum standardized uptake value 5.4) (b) Transaxial fused contrast enhanced positron emission tomography-computed tomography images showing dual time point imaging which shows increase in maximum standardized uptake value from 4.4 to 5.7 in left lingual tonsil, with no corresponding computed tomography abnormality. (c) Coronal and transaxial contrast-enhanced computed tomography and corresponding fused positron emission contrast-enhanced computed tomography images of a right hand showing focus of fluorodeoxyglucose uptake in soft tissue in the distal forearm between radius and ulna. Histopathological examination of tonsillectomy specimen - reactive lymphoid follicles. (C) 68Gallium-DOTANOC contrast enhanced positron emission tomography-computed tomography images showing a). physiological tracer distribution and focus of abnormal increase in tracer in region of greater trochanter of right femur, (b) Fused contrast enhanced positron emission tomography-computed tomography and (c) corresponding contrast-enhanced computed tomography axial, sagittal and coronal images showing focal increase in tracer uptake in greater trochanter of right femur adjacent to bone chips. The patient underwent bilateral total hip replacement. (d and e) Histopathological examination showed atypical spindle cell lesion. Postoperative serum calcium – 10 mg/dL, serum phosphorus – 2 mg/dL
DISCUSSION
TIO is an important cause of adult onset hypophosphatemic osteomalacia. Patients with TIO exhibit clinical hallmarks of osteomalacia, including bone and muscle pain, severe muscle weakness, gait disturbances, and heightened susceptibility to fractures. These tumors produce fibroblast growth factor (FGF-23), a hormone that inhibits renal phosphate reabsorption and renal production of 1,25-(OH) 2D3.
The mesenchymal tumors associated with oncogenic osteomalacia have, till date, been reported under various names including hemangiopericytoma, sclerosing hemangiomas and angiolipoma.[2] The term phosphaturic mesenchymal tumors, mixed connective tissue variant “PMTCMT” was proposed as a unifying concept by Weidner and Santa Crux in 1987.[3] However, this concept has still not been brought in common usage. The histologic appearance of PMTCMT comprises of the bland spindle or stellate cells embedded in a smudgy matrix, which may undergo calcification. The bland spindle cells express FGF-23. The most common histology reported in our study was hemangiopericytoma (5/8). The other histologies reported include phosphaturic mesenchymal tumors in 2/8 patients and angiofibroma in 1/8 patient.
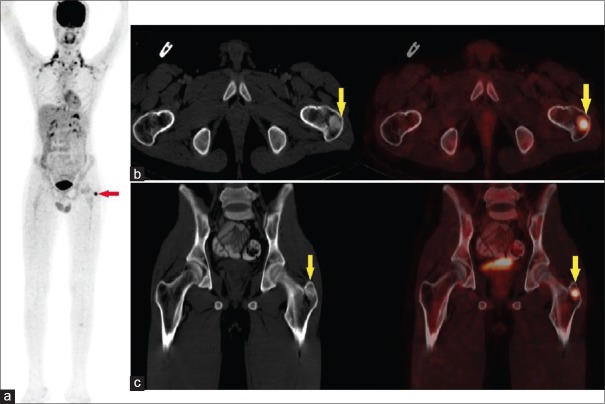
Approximately, 53% of PMTCMTs occur in bone, 45% in soft tissue and 3% in the skin.[4] PMTCMT most common involves extremities while craniofacial localization has been described in only 5% of cases.[2] In our study, tumor was located in bone in 2/8 patients [Figure 3A–C] and in the craniofacial region in 6/8 patients. Surgery remains the mainstay of treatment of these tumors. Quite a dramatic normalization of biochemical abnormalities is observed within days to weeks following successful resection of the tumor.[2] Similar improvement in symptoms was observed in our patients except one.
Figure 3.
52/M with difficulty in walking since 7 months and proximal myopathy since 7 years. Preoperative biochemical parameters: Serum calcium - 7.5 mg/dL, serum phosphorus – 0.7 mg/dL serum parathyroid hormone – 277.4 pg/ml, serum creatinine – 1.3 mg/dL, serum alkaline phosphatase – 1093 U/L. Fluorodeoxyglucose contrast enhanced positron emission tomography-computed tomography images (a) maximum intensity projection image showing brown fat uptake and focus of increased fluorodeoxyglucose uptake in the region of greater trochanter of left femur (arrow). (b) Transaxial and (c) coronal contrast-enhanced computed tomography and corresponding fused contrast enhanced positron emission tomography-computed tomography showing fluorodeoxyglucose uptake in focal sclerotic lesion in the greater trochanter of left femur (maximum standardized uptake value 6.9). Postoperative serum calcium – 9.4 mg/dL, serum phosphorus 2.8 m/dL
Various modalities used for tumor localization include radiographs, ultrasonography, CECT, MRI, whole body technetium (Tc) bone scan, FDG PET-CT and octreotide scintigraphy[5,6,7] and recently 68Ga DOTATATE PET-CT. Functional imaging should be performed first in the diagnostic pathway of localization of tumor causing TIO. 99mTc-methylene diphosphonate (99mTc-MDP) whole body bone scan usually demonstrates typical findings of osteomalacia, that is, widening of mandible, rachitic rosary sign, sign of sternum, pseudofracture, and prominent epiphyses of knees suggesting it to be metabolic in nature.[8] It detects the involved bones in osteomalacia including fractures rather than the primary tumor itself. However, MDP bone scans show uptake at the costochondral junctions and areas of bone in skeletally mature adults where the growth plates were previously located. This finding should suggest the diagnosis of TIO, and may represent a sort of “pseudo-reactivation” of growth plates in the adult skeleton.[9] The pathophysiology underlying this frequently observed phenomenon is unclear at this time.
Dupond et al. in his case report emphasized that FDG PET-CT in conjunction with FGF-23 levels is an important decisive tool in the management of oncogenic osteomalacia.[10] As these tumors are very slow growing and small in size, the uptake of FDG may be low. Recent literature suggests that they variably express SSTRs.[11] These characteristics have aroused interest in SSTR imaging in these patients. Agrawal et al. in his retrospective analysis of six patients showed that 68Ga DOTATATE PET-CT detected tumor in 5/6 patients (83.3%) and concluded that 68Ga DOTATATE PET-CT performed better than FDG PET-CT in detection of tumors causing oncogenic osteomalacia.[12] Similarly, Clifton-Bligh et al. in his analysis of six patients showed that 68Ga DOTATATE PET-CT was able to detect a tumor in 5/6 patients with confidence.[13] Jadhav et al. in his study (n = 16) showed positivity rate of 50% with FDG PET-CT and 100% with 68Ga DOTATATE PET-CT.[14] Similarly Chong et al. (n = 31) in his study, showed sensitivity of FDG PET-CT to be 88% and OCTREO-SPECT to be 95%.[15] The sensitivity of FDG PET-ceCT in our study was high (87.5%). It may be attributed to the time of flight technology inbuilt in our machine, which enables small lesion detection and also use of contrast for diagnostic quality CT images. FDG PET-CT is highly sensitive, but it is nonspecific as it may show areas of increased metabolic activity in sites of active fracture healing. Low-grade uptake is also seen in the fractures in 68Ga DOTATATE PET-CT as the osteoblasts also express SSTR.[16]
We emphasize the role of FDG PET-ceCT in the localization of tumors in a patient with TIO in this study. Whole body imaging inclusive of the upper and lower limbs is mandatory since these tumors are located in obscure sites. Once the site of tumor is localized, regional MRI can be done to characterize the soft tissue lesion. Ga DOTANOC PET-CT is valuable in diagnosing tumor as they express SSTRs. However, due to lack of availability at each center, FDG PET-CT which is widely available may be used as the initial imaging modality. If FDG PET-ceCT is unable to detect the site, then SSTR imaging can be performed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Weiss D, Bar RS, Weidner N, Wener M, Lee F. Oncogenic osteomalacia: Strange tumours in strange places. Postgrad Med J. 1985;61:349–55. doi: 10.1136/pgmj.61.714.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Folpe AL, Fanburg-Smith JC, Billings SD, Bisceglia M, Bertoni F, Cho JY, et al. Most osteomalacia-associated mesenchymal tumors are a single histopathologic entity: An analysis of 32 cases and a comprehensive review of the literature. Am J Surg Pathol. 2004;28:1–30. doi: 10.1097/00000478-200401000-00001. [DOI] [PubMed] [Google Scholar]
- 3.Weidner N, Santa Cruz D. Phosphaturic mesenchymal tumors. A polymorphous group causing osteomalacia or rickets. Cancer. 1987;59:1442–54. doi: 10.1002/1097-0142(19870415)59:8<1442::aid-cncr2820590810>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 4.Ungari C, Rocchi G, Rinna C, Agrillo A, Lattanzi A, Pagnoni M. Hypophosphaturic mesenchymal tumor of the ethmoid associated with oncogenic osteomalacia. J Craniofac Surg. 2004;15:523–7. doi: 10.1097/00001665-200405000-00036. [DOI] [PubMed] [Google Scholar]
- 5.Fukumoto S, Takeuchi Y, Nagano A, Fujita T. Diagnostic utility of magnetic resonance imaging skeletal survey in a patient with oncogenic osteomalacia. Bone. 1999;25:375–7. doi: 10.1016/s8756-3282(99)00170-2. [DOI] [PubMed] [Google Scholar]
- 6.Hodgson SF, Clarke BL, Tebben PJ, Mullan BP, Cooney WP, 3rd, Shives TC. Oncogenic osteomalacia: Localization of underlying peripheral mesenchymal tumors with use of Tc 99m sestamibi scintigraphy. Endocr Pract. 2006;12:35–42. doi: 10.4158/EP.12.1.35. [DOI] [PubMed] [Google Scholar]
- 7.Jan de Beur SM, Streeten EA, Civelek AC, McCarthy EF, Uribe L, Marx SJ, et al. Localisation of mesenchymal tumours by somatostatin receptor imaging. Lancet. 2002;359:761–3. doi: 10.1016/s0140-6736(02)07846-7. [DOI] [PubMed] [Google Scholar]
- 8.Sood A, Agarwal K, Shukla J, Goel R, Dhir V, Bhattacharya A, et al. Bone scintigraphic patterns in patients of tumor induced osteomalacia. Indian J Nucl Med. 2013;28:173–5. doi: 10.4103/0972-3919.119541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chong WH, Molinolo AA, Chen CC, Collins MT. Tumor-induced osteomalacia. Endocr Relat Cancer. 2011;18:R53–77. doi: 10.1530/ERC-11-0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dupond JL, Mahammedi H, Magy N, Blagosklonov O, Meaux-Ruault N, Kantelip B. Detection of a mesenchymal tumor responsible for hypophosphatemic osteomalacia using FDG-PET. Eur J Intern Med. 2005;16:445–6. doi: 10.1016/j.ejim.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 11.Auethavekiat P, Roberts JR, Biega TJ, Toney MO, Christensen RS, Belnap CM, et al. Case 3. Oncogenic osteomalacia associated with hemangiopericytoma localized by octreotide scan. J Clin Oncol. 2005;23:3626–8. doi: 10.1200/JCO.2005.05.043. [DOI] [PubMed] [Google Scholar]
- 12.Agrawal K, Bhadada S, Mittal BR, Shukla J, Sood A, Bhattacharya A, et al. Comparison of 18F-FDG and 68Ga DOTATATE PET/CT in localization of tumor causing oncogenic osteomalacia. Clin Nucl Med. 2015;40:e6–10. doi: 10.1097/RLU.0000000000000460. [DOI] [PubMed] [Google Scholar]
- 13.Clifton-Bligh RJ, Hofman MS, Duncan E, Sim IeW, Darnell D, Clarkson A, et al. Improving diagnosis of tumor-induced osteomalacia with Gallium-68 DOTATATE PET/CT. J Clin Endocrinol Metab. 2013;98:687–94. doi: 10.1210/jc.2012-3642. [DOI] [PubMed] [Google Scholar]
- 14.Jadhav S, Kasaliwal R, Lele V, Rangarajan V, Chandra P, Shah H, et al. Functional imaging in primary tumour-induced osteomalacia: Relative performance of FDG PET/CT vs somatostatin receptor-based functional scans: A series of nine patients. Clin Endocrinol (Oxf) 2014;81:31–7. doi: 10.1111/cen.12426. [DOI] [PubMed] [Google Scholar]
- 15.Chong WH, Andreopoulou P, Chen CC, Reynolds J, Guthrie L, Kelly M, et al. Tumor localization and biochemical response to cure in tumor-induced osteomalacia. J Bone Miner Res. 2013;28:1386–98. doi: 10.1002/jbmr.1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hofman MS, Hicks RJ. Changing paradigms with molecular imaging of neuroendocrine tumors. Discov Med. 2012;14:71–81. [PubMed] [Google Scholar]