Abstract
Background:
Probiotic delivery systems are widely used nutraceutical products for the supplementation of natural intestinal flora. These delivery systems vary greatly in the effectiveness to exert health benefits for a patient. This study focuses on providing probiotic living cells with a physical barrier against adverse environmental conditions.
Materials and Methods:
Microencapsulation of the selected lactic acid bacteria (LAB) using chitosan and alginate was performed. Physical examination of the formulated LAB microcapsules was observed using phase contrast inverted microscope and scanning electron microscope (SEM). Finally, the survival of microencapsulated and noncapsulated bacteria was cheeked in the simulated human gastric tract (GT). The potential antimicrobial activity of the most potent microencapsulated LAB strain was in vivo evaluated in rabbit models.
Results:
Microencapsulated L. plantarum, L. acidophilus, and L. bulgaricus DSMZ 20080 were loaded with 1.03 × 1010 CFU viable bacteria/g, 1.9 × 1010 CFU viable bacteria/g, and 5.5 × 109 CFU viable bacteria/g, respectively. The survival of microencapsulated cells was significantly higher than that of the free cells after exposure to simulated gastric juice (SGJ) at pH 2. Additionally, in simulated small intestine juice (SSJ), larger amounts of the selected LAB cells were found, whereas in simulated colon juice (SCJ), the released LAB reached the maximum counts. In vivo results pointed out that an 8-week supplementation with a triple therapy of a microencapsulated L. plantarum, L. acidophilus, and L. bulgaricus DSMZ 20080 might be able to reduce H. pylori.
Conclusion:
Microencapsulated probiotics could possibly compete with and downregulate H. pylori infection in humans.
Keywords: Helicobacter pylori (H. pylori), microcapsule, probiotics
INTRODUCTION
Helicobacter pylori (H. pylori) is one of the most prevalent pathogens as more than 50% of the world population is colonized by this microorganism, low socioeconomic level and bad hygienic conditions being the main risk factors.[1] Gastric colonization by H. pylori results in chronic gastritis, which will remain asymptomatic in most subjects who consequently may not be treated with antibiotics to eradicate it. A new treatment regimen that would achieve the eradication rates of 90% or greater seen at the advent of H. pylori treatment is urgently needed.[2] Multiple regimens have been evaluated for H. pylori therapy in randomized controlled trials[3] including triple therapy with a proton pump inhibitor (PPI).[4] Quadruple therapy consists of a PPI, combined with bismuth and two antibiotics (e.g., metronidazole 250 mg four times daily and tetracycline 500 mg four times daily) given for 10-14 days,[5] an open label prospective trial, H. pylori treatment-naïve patients randomized to levofloxacin, omeprazole, nitazoxanide, and doxycycline (LOAD) for 7 days or 10 days,[6] and sequential therapy.[7] Despite the number of studies, the optimal therapeutic regimen has not yet been defined. The treatment regimen that is selected must be effective but considerations such as cost, side effects, and ease of administration should also be taken into account.[1,4,7,8,9,10] Eradication may be confirmed by a urea breath test, fecal antigen test, or upper endoscopy performed 4 weeks or more after completion of therapy.[11] Confirmation of eradication should be strongly considered for all patients receiving treatment for H. pylori because of the availability of accurate, relatively inexpensive, noninvasive tests (stool and breath tests), and increasing antibiotic resistance.[5] Recently, using of probiotics in H. pylori-colonized subjects with gastric inflammation is supported by many observations.[12,13] Probiotic delivery systems are widely used nutraceutical products for the supplementation of natural intestinal flora. These delivery systems vary greatly in the effectiveness to exert health benefits on a patient. Probiotic delivery systems can be categorized into conventional, pharmaceutical formulations, and nonconventional, mainly commercial food-based products. Conventional pharmaceutical products tend to be more effective in this regard and are much more characterized compared to commercial food-based carrier systems. Examples of pharmaceutical formulations for the delivery of probiotics currently include, among others, beads, capsules, and tablets.[14,15]
In the development of effective and safe encapsulated products, it is essential to maintain the adequate number of viable cells during the shelf life of the product as well as during the gastric tract (GT) after consumption.[16,17] Microencapsulation will assume importance in delivering viable strains of probiotic bacteria in large numbers to consumers.[18] The main factors affecting the viability of probiotics in the GT are the acidic environments that present a challenge for the application of these microorganisms in different industries.[19] To overcome difficulty during development, microencapsulation technique is utilized to increase the viabilities of probiotics.[20,21,22] Some specific strains of Lactobacillus and Bifidobacterium inhibit H. pylori through the production of bacteriocins or organic acids.[23,24] Probiotics may exert some of their protective functions through the modulation of immune activity and epithelial functions in both the large and small intestines.[25]
Our study aimed to formulate microencapsulated LAB and evaluated their survival in the simulated human GT.
MATERIALS AND METHODS
Used probiotics
The most potent anti-H. pylori, anti-inflammatory, and antioxidant lactic acid bacteria (LAB) strains were selected (Lactobacillus plantarum, Lactobacillus acidophilus, and Lactobacillus bulgaricus DSMZ 20080) as previously described by El-Adwai.[26,27] The selected probiotic cells were collected from de Man, Rogosa, and Sharpe (MRS) broth culture in the early stationary phase of growth at 37°C under aerobic and anaerobic conditions. Cells were washed three times with a sterile phosphate buffer solution (PBS, pH = 7.4), suspended in a sterile peptone water solution and kept at − 80°C for 24-48 h, and then lyophilized.
Microencapsulation of probiotics
The microencapsulation of the selected probiotics were prepared according to the method described by Krasaekoopt et al.[28] Briefly, 5 g of lyophilized LAB was mixed with 20 mL of 20 g/L sodium presterilized alginate solution. The sterile syringe was filled with the cell suspension and was injected through a 0.11-mm needle (Nisco, Switzerland) by a syringe bump adjusted at rate 0.27 into sterile 0.05 mol/L CaCl2 containing 0.1 g/100 mL Tween 80. After 30 min of gelification in 0.05 mol/L CaCl2, the beads were rinsed and kept in a sterile peptone water solution at 4°C. Low-molecular-weight chitosan (0.5 g) dissolved in 50 mL 1% acetic acid solution (adjusted according to LAB growth tolerance); the pH was adjusted in a range of 5.7 to 6.0 followed by sterilization at 121°C for 15 min. Fifteen grams of washed beads were immersed in 100 mL of chitosan solution with gentle shaking at 100 rpm for 40 min on an orbital shaker (Thermo Scientific, USA) for coating (two-step method). The chitosan-coated beads were rinsed and washed twice with sterilized peptone water solution. The morphological and microstructural of obtained beads were examined using phase contrast inverted microscope. The beads were suspended in peptone water solution, maintained at −80°C for 24-48 h, and then lyophilized and stored at 2°C.
Sampling of microcapsules for microstructural analysis by scanning electron microscope
Microcapsules were prepared and examined under a scanning electron microscope (SEM) (model JSM-5200LV, Jeol Datum Co., Japan) as described by Rosenberg et al.[29]
The viability of lactic acid bacteria in formulated microcapsules
The microcapsules (1 g) were liquefied in sterile sodium citrate 1% solution (pH 6.0) and incubated in a shaker incubator (New Brunswich, USA) at 37°C for 2 h.[30] MRS agar medium was inoculated with different dilutions of prepared microcapsule solutions and incubated at 37°C for 48 h. The colony-forming units of survived bacteria were estimated. The experiments were replicated three times.
Simulation of the human gastrointestinal environment
The used human gastrointestinal (GI) conditions were anaerobically simulated at 37°C in vitro as described by Chen et al.[31]
Food content of human western diet suspension
It is composed of (per liter) 1 g arabinogalactan, 2 g pectin, 1 g xylan, 3 g potato starch, 0.4 g glucose, 3 g yeast extract, 1 g peptone, 4 g mucin, and 0.5 g cystein.
Simulated gastric juice (SGJ)
Food content suspension (30 mL) was sterilized by filtration and acidified to pH ≤2 in closed sterilized vessels.
Simulated small intestine juice (SSJ)
Sterilized food suspension (30 mL) was neutralized (pH ≥6.8) with the addition of simulated pancreatic juice (0.9 g pancreatin, 6 g bile salts, and 12 g NaHCO3 per liter).
Simulated colon juice (SCJ)
Food suspension was transferred to the simulated colon with pH value 6.2-6.8.
The survival of microencapsulated bacteria in the simulated human gastrointestinal tract
The survival of microencapsulated and noncapsulated bacteria was cheeked in the simulated human gastrointestinal tract (GIT) as described by Chen et al.[31] and Berrada et al.[32] Briefly, microcapsules (1 g) and noncapsulated bacterial suspension (1 mL; 2 × 105) was exposed to the simulated human GI fluids. After exposure time, the viability of LAB in simulated human GI fluids was determined as mentioned above.[30] After transit stages, microcapsule samples were withdrawn for morphological examination under an inverted microscope (Olympus, USA).
Statistical analyses
Statistical analysis of the present study was conducted, using the mean, standard error, and t-tests by the Statistical Package for the Social Sciences (SPSS) version 16 (SPSS Inc., Chicago, IL). The normality of data was measured using Shapiro-Wilk test by SPSS version 16.[33,34] In all cases, a P value of <0.05 was considered to be statistically significant.
RESULTS
The early stationary phase of the selected strains, L. plantarum, L. acidophilus, and L. bulgaricus DSMZ 20080 were ranged 29-19 h (data not shown). The selected strains were microencapsulated in two polysaccharide layers (alginate and chitosan) and they were able to tolerate chitosan dissolved in 1% acetic acid.
Physical examination of formulated microcapsules
Using phase contrast inverted microscope, microcapsules appeared as teardrop-shaped with a double layer; the chitosan outer layer and lactic acid bacteria embedded in the alginate inner layer [Figure 1]. The microcapsules were observed under SEM as spherical and wrinkled surface beads as shown in Figure 2.
Figure 1.
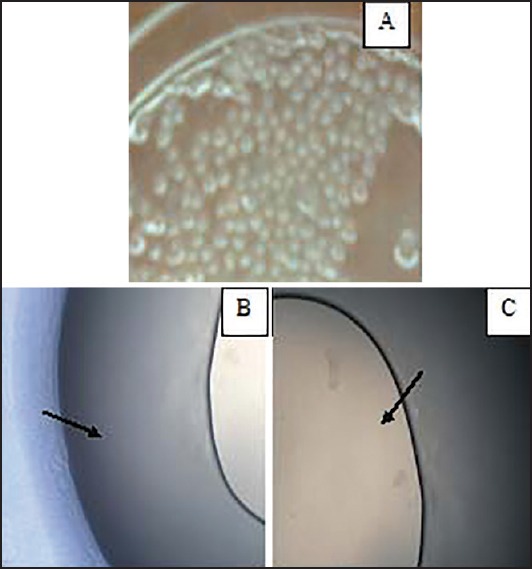
Phase contrast inverted microscope of microcapsules. (a) The teardrop-shaped microcapsules (b and c) The double layer microcapsules. The small dark arrows in B point to the outer chitosan layer while in C point to the inner alginate layer
Figure 2.
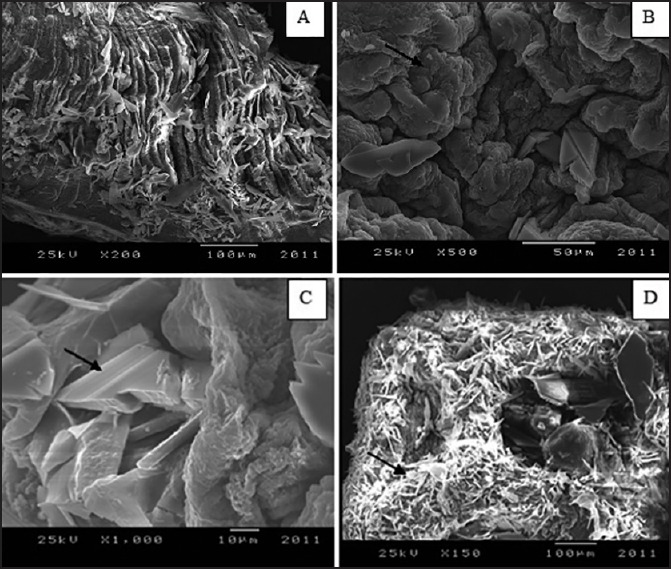
Scanning electron microscope of the microcapsules. (a) The whole microcapsules of lactic acid bacteria showing the double layer alginate and chitosan (b and c) The chips chitosan layer covered and impeded in the porous alginate layer at high magnification power (d) The internal half of microcapsules. The small dark arrows in B point to the porous alginate layer while in C, the arrow points to the chips of the chitosan layer and arrow points to lactic acid bacteria within the alginate matrix in D
Using SEM, the inner half of the two halves of the microcapsule was scanned as shown in Figure 2. Bacteria were found to be entrapped in the groups that were randomly distributed within the alginate matrix of the microcapsule [Figure 2].
Viability of formulated lactic acid bacteria microcapsules
The obtained results indicated that each L. plantarum, L. acidophilus, and L. bulgaricus DSMZ 20080 microencapsulated were loaded with 1.03 × 1010CFU viable bacteria/g, 1.9 × 1010 CFU viable bacteria/g, and 5.5 × 109 CFU viable bacteria/g, respectively. Microcapsulated bacteria were exposed to the simulated GI media and compared with the noncapsulated one as observed in Table 1.
Table 1.
Survival of microcapsulated bacteria in the simulated human GIT
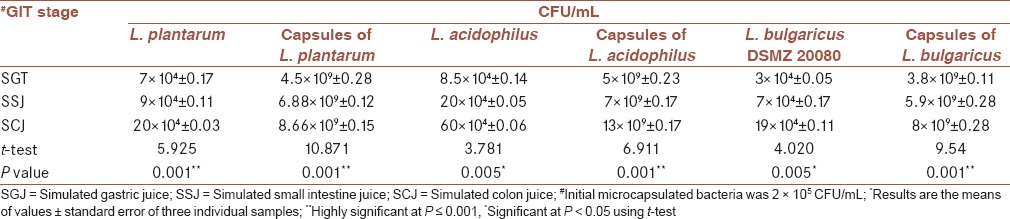
Generally, it was clarified that the surviving of microencapsulated cells was significantly higher than that of the free cells after exposure to SGJ at pH 2, whereas the release amounts of L. plantarum, L. acidophilus, and L. bulgaricus DSMZ 20080 cells were 4.5 × 109 CFU/mL, 5 × 109 CFU/mL, and 3.8 × 109 CFU/mL, respectively, compared with this noncapsulated one that recorded 7 × 104 CFU/mL, 8.5 × 104 CFU/mL, and 3 × 104 CFU/mL, respectively. In addition, in SSJ larger amounts of the selected LAB cells (6.88 × 109 CFU/mL, 7 × 109 CFU/mL, and 5.9 × 109 CFU/mL, for L. plantarum, L. acidophilus, and L. bulgaricus DSMZ 20080, respectively) were found. With regard to SCJ, the released LAB reached the maximum; L. plantarum, L. acidophilus, and L. bulgaricus DSMZ 20080 cells recorded 8.66 × 109 CFU/mL, 13 × 109 CFU/mL, and 8 × 109 CFU/mL, respectively compared with the noncapsulated strains that counted 2 × 105 CFU/mL, 6 × 105 CFU/mL, and 1.9 × 105 CFU/mL, respectively [Table 1]. There was a highly statistically significant difference in the survival of the number of capsulated and noncapsulated bacteria (P value = 0.001) as represented in Table 1. The normality of data was measured using a Shapiro-Wilk test (P > 0.05) and a visual inspection of their histograms, normal Q-Q plots showed the survival of microcapsulated bacteria (CFU/mL) in the simulated human GIT were approximately normally distributed for different GIT stages (SGT, SSJ, and SCJ), with a skewness of 0.885 (SE = 0.913) and a Kurtosis of −1.745 (SE = 2.000) for SGT, a skewness of 0.096 (SE = 0.845), and a Kurtosis of −3.068 (SE = 1.714) for SSJ and a skewness of 0.044 (SE = 0.845) and a Kurtosis of −3.218 (SE = 1.741) for SCJ.
The survival of the microcapsulated bacteria in the simulated human GIT was photographed [Figure 3].
Figure 3.
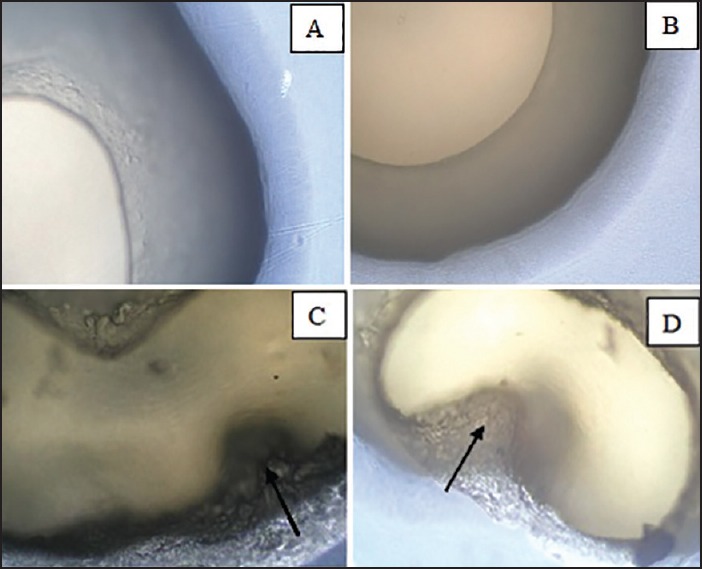
The survival of microencapsulated bacteria in the simulated human GIT. (a) The double layers of the microcapsules were intact in the initial stage (b) The outer chitosan layer was shrunken and decreased in the size in the SGJ stage (c and d) Both the chitosan and alginate layers were disrupted in the SSJ stage and SCJ stage, respectively. The dark arrow in C points to the disrupted alginate while in D the arrow points to the disrupted chitosan layers
In vivo efficacy of microencapsulated lactic acid bacteria to inhibit H. pylori
The potential antimicrobial activity of the most potent microencapsulated LAB strain was in vivo evaluated. Histology of pyloric and subpyloric mucosa are illustrated in Figures 4 and 5, respectively, for control group (CG), infected group (IG), and treated group (TG) in rabbits.
Figure 4.
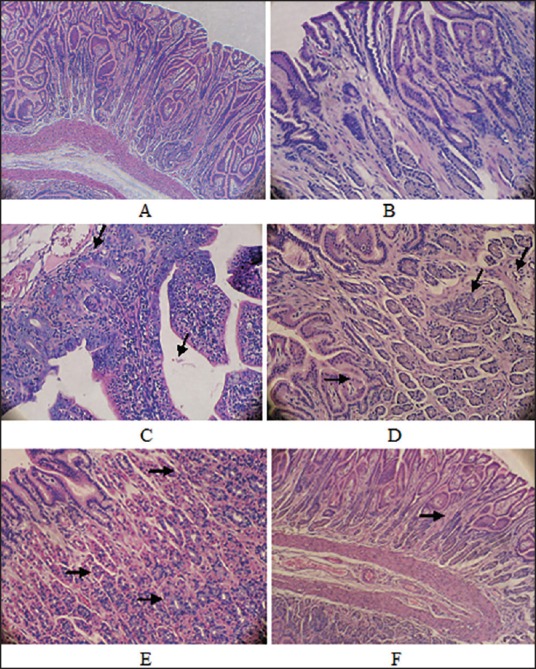
Histology of pyloric mucosa's (H and E, ×40). (a) Control group (b-e) Infected groups (b) Discontinuity of the epithelium, interstitial edema, widening of the bile duct, and congested blood capillary (c) The cellular infiltration, disorganization, reactive atypia and congested blood (d) The duct wider, multiple vaculations, interstitial edema between glands and mononeuclear cell infiltration (e) Cytoplasmic acidophilic increased (f) Treated groups showing a decrease in the widening of the bile duct and in cellular infiltration and the absence of inflammatory cells
Figure 5.
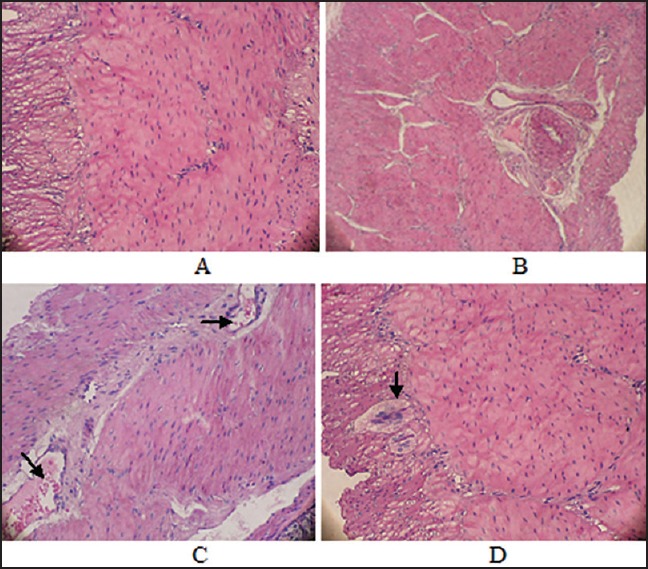
Histology of pyloric submucosas (H&E, ×40). (a) Control group (b and c) Infected groups (b) Dilated congested blood vesicles with cellular infiltration and edematous aria between muscles (fibrosis) (c) Cellular infiltration increased (d) Treated group showing nerve hyperplasia and a decrease in dilation of the blood vesicles and disappearance of cellular infiltration
In order to evaluate the potential antimicrobial activity of the most potent encapsulated LAB strain in vivo, 12 healthy albino male rabbits were used. Generally, the infected rabbits developed chronic active gastritis closely resembling the H. pylori gastritis observed in humans. In animals from non- H. pylori inoculated groups (CG), inflammatory cell infiltration into the lamina propria was negligible. In contrast, active atrophic gastritis, reactive atypia, and multiple vacuoles developed in the gastric mucosa of the rabbits in the infected group (IF), which thus showed a marked mucosal infiltration of inflammatory cells in the lamina propria. In addition, superficial erosions in the antrum and irregularity of the pyloric glands were frequently observed [Figures 4b and e and 5a-c]. However, the degree of mucosal infiltration of inflammatory cells [all of intraepithelial lymphocytes (IELs), polymorphonuclear and mononuclear cells] in the TG was significantly less than in the CG [Figures 4f and 5d]. In addition, no intestinal metaplasia or tumors in the gastric mucosa were observed in any groups based on the findings of this experiment.
Generally, in vivo results pointed out that an 8-week supplementation with a triple therapy of a microencapsulated L. plantarum, L. acidophilus, and L. bulgaricus DSMZ 20080 may be able to reduce H. pylori and their pathogenicity in experimental animals and control gastric inflammation.
DISCUSSION
Emergence of drug resistance in H. pylori is a major obstacle in the eradication of this gastric pathogen.[32] The application of probiotic organisms in food preservation as well as prevention of human GI diseases is emerging these days.
Providing probiotic living cells with a physical barrier against adverse environmental conditions is therefore, an approach currently of considerable interest.[35] Thus, the encapsulation technique has been applied to increase the survival and delivery of bacterial cultures. The purpose of microencapsulation of probiotics is to protect certain compounds or biological cells against the surrounding environment, which destruct the core. It protects the bacteria from heat, oxygen, and moisture and also improves the flow properties during formulation development. It can be used for different drug delivery systems and at present to apply for the encapsulation of probiotics in food products.[35,36,37,38,39] The core material is encapsulated in the food grade matrix type coating material. In the food industry, these materials form a barrier to protect the core material against the GI environment using different encapsulation systems.[39]
For the successful exploitation of microcapsules as an oral delivery device, an understanding of their performance under physiologically pertinent conditions that represent the human GIT is essential. Although in vivo research using specific techniques such as histological sectioning,[40] radiography,[41] and gamma scintigraphy[42] can progressively track the microcapsules in the GIT, it remains difficult to follow the orally administered microcapsule at every stage of digestion in either animals or humans due to tedious processing, small size of the microcapsules, limited detection resolution, and ethical constraints. In vitro simulation offers a number of advantages, for example, well-controlled experimental conditions and easy sampling, especially preferable for screening and examining a variety of samples. Buffered solutions, for example, with pH at 1-2 or 6.5-7.5, are frequently reported in the literature as the simulated GI fluids;[43] however they only represent the pH in the stomach and in the intestine, and do not mimic the complex human GI microbial ecosystem. While other ex vivo and in vitro simulated models including USP apparatus were also reported,[44] most of them were static systems where fluids cannot be continuously transferred into the sequential GI compartments.
Our study uses a dynamic controlled human simulated GI model. Microcapsules maintain physical integrity in the GIT to prevent the leaking of genetically engineered cells, which is strongly dependent on the microcapsules’ robustness and stability. So far, few studies have been reported to address this matter.[45] Certain lactobacilli are resistant to the low pH of the stomach and may adhere to and transiently reside in the human stomach.[46] The most frequently used strain was L. johnsonii La1, either in a fermented milk preparation containing live bacteria[47] or as a free-cell culture supernatant.[48] Other probiotics used were L. casei,[49] L. brevis,[50] and L. gasseri OLL2716.[51]
Microcapsules are made of various materials and chemicals through complexation and cross-linking reactions, all of which may have an effect on the biocompatibility of the final microcapsules. In particular, the well-balanced gut microbiota is important in maintaining human health[52] and should not be altered by the intake of microcapsules. Our results suggested that the used materials to construct the microcapsules did not evoke appreciable adverse effects on the human intestinal flora. In addition, cross-linked chitosan membranes did not compromise the biocompatibility of the microcapsules comparing to the noncross-linked subjects. These results were comparable to the results of Bhatia et al.[45] and Zimmermann et al.[53] The well-balanced gut microbiota is important in maintaining human health and should not be altered by the intake of microcapsules.[52] It could possibly be attributed to the binding effect or diffusion of the enzymes to the microcapsules though further research may continue to elucidate the consequence.
The adhesion of H. pylori to epithelial cells is important in determining the outcome in H. pylori-associated diseases. Our results clarified that in vivo treatment with microencapsulated L. plantarum, L. acidophilus, and L. bulgaricus DSMZ 20080 have an inhibitory effect on H. pylori. In the gastric mucosa, H. pylori possibly interacts with epithelial cells through secretory components or as a result of adherence.[54] Furthermore, several studies showed that L. johnsonii La1, L. salivarius, L. acidophilus, and W. confusa inhibit the attachment of H. pylori to intestinal HT-29 cells[55] or to MKN 45 gastric cell lines.[56] Johnson-Henry[57] demonstrated that previous colonization by probiotics prevented or reduced H. pylori infection in germ-free mice. In addition, L. salivarius suppressed H. pylori and reduced the inflammatory response in gnotobiotic mice more efficiently than L. acidophilus or L. casei.[58] It has been suggested that the degree of suppression of H. pylori depends on the used probiotic strain.
CONCLUSION
In conclusion, in vitro studies revealed that microencapsulated probiotics could possibly compete with and downregulate H. pylori infection in humans.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
AUTHOR'S CONTRIBUTION
MAK contributed in the conception of the work, conducting the study, revising the draft, approval of the final version of the manuscript, and agreed for all aspects of the work. MME contributed in the conception of the work, drafting and revising the draft, approval of the final version of the manuscript, and agreed for all aspects of the work. HIE contributed in the conception of the work, conducting the study, revising the draft, approval of the final version of the manuscript, and agreed for all aspects of the work. NME contributed in the conception of the work, revising the draft, approval of the final version of the manuscript, and agreed for all aspects of the work. MZH contributed in approval of the final version of the manuscript including acquisition and agreed for all aspects of the work.
Acknowledgements
The authors thank Dr. Hanan Sloiman and Dr. Asem Elfert (Department of Tropical Medicine and Infectious Diseases, Faculty of Medicine, Tanta University) for kindly providing the biopsy specimens and Dr. Nagla Sarhan (Department of Histopathology, Faculty of Medicine, Tanta University) for helping with the preparation and examination of histological samples.
They also wish to thank Dr. Gamal El Maghraby (Department of Pharmaceutical technology, Faculty of Pharmacy, Tanta University) for helping in the preparation of microcapsules.
REFERENCES
- 1.Hunt RH, Xiao SD, Megraud F, Leon-Barua R, Bazzoli F, van der Merwe S, et al. Helicobacter pylori in developing countries. World Gastroenterology Organization Global Guidelines. J Gastrointestin Liver Dis. 2011;20:299–304. [PubMed] [Google Scholar]
- 2.Weizman Z, Asli G, Alsheikh A. Effect of a probiotic infant formula on infections in child care centers: Comparison of two probiotic agents. Pediatrics. 2005;115:5–9. doi: 10.1542/peds.2004-1815. [DOI] [PubMed] [Google Scholar]
- 3.Qasim A, Sebastian S, Thornton O, Dobson M, McLoughlin R, Buckley M, et al. Rifabutin- and furazolidone-based Helicobacter pylori eradication therapies after failure of standard first- and second-line eradication attempts in dyspepsia patients. Aliment Pharmacol Ther. 2005;21:91–6. doi: 10.1111/j.1365-2036.2004.02210.x. [DOI] [PubMed] [Google Scholar]
- 4.Gisbert JP, Gonzalez L, Calvet X. Systematic review and meta-analysis: Proton pump inhibitor vs.ranitidine bismuth citrate plus two antibiotics in Helicobacter pylori eradication. Helicobacter. 2005;10:157–71. doi: 10.1111/j.1523-5378.2005.00307.x. [DOI] [PubMed] [Google Scholar]
- 5.McColl KE. Clinical practice. Helicobacter pylori infection. N Engl J Med. 2010;362:1597–604. doi: 10.1056/NEJMcp1001110. [DOI] [PubMed] [Google Scholar]
- 6.Basu PP, Rayapudi K, Pacana T, Shah NJ, Krishnaswamy N, Flynn M. A randomized study comparing levofloxacin, omeprazole, nitazoxanide, and doxycycline versus triple therapy for the eradication of Helicobacter pylori. Am J Gastroenterol. 2011;106:1970–5. doi: 10.1038/ajg.2011.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moayyedi P, Malfertheiner P. Editorial: Sequential therapy for eradication of Helicobacter pylori: A new guiding light or a false dawn? Am J Gastroenterol. 2009;104:3081–3. doi: 10.1038/ajg.2009.563. [DOI] [PubMed] [Google Scholar]
- 8.Gatta L, Zullo A, Perna F, Ricci C, De Francesco V, Tampieri A, et al. A 10-day levofloxacin-based triple therapy in patients who have failed two eradication courses. Aliment Pharmacol Ther. 2005;22:45–9. doi: 10.1111/j.1365-2036.2005.02522.x. [DOI] [PubMed] [Google Scholar]
- 9.Fischbach LA, van Zanten S, Dickason J. Meta-analysis: The efficacy, adverse events, and adherence related to first-line anti-Helicobacter pylori quadruple therapies. Aliment Pharmacol Ther. 2004;20:1071–82. doi: 10.1111/j.1365-2036.2004.02248.x. [DOI] [PubMed] [Google Scholar]
- 10.Graham DY, Hammoud F, El-Zimaity HM, Kim JG, Osato MS, El-Serag HB. Meta-analysis: Proton pump inhibitor or H2-receptor antagonist for Helicobacter pylori eradication. Aliment Pharmacol Ther. 2003;17:1229–36. doi: 10.1046/j.1365-2036.2003.01583.x. [DOI] [PubMed] [Google Scholar]
- 11.Chey WD, Wong BC Practice Parameters Committee of the American College of Gastroenterology. American College of Gastroenterology guideline on the management of Helicobacter pylori. Am J Gastroenterol. 2007;102:1808–25. doi: 10.1111/j.1572-0241.2007.01393.x. [DOI] [PubMed] [Google Scholar]
- 12.Yaşar B, Abut E, Kayadıbı H, Toros B, Sezıklı M, Akhan Z, et al. Efficacy of probiotics in Helicobater pylori eradication therapy. Turk J Gastroenterol. 2010;21:212–7. doi: 10.4318/tjg.2010.0090. [DOI] [PubMed] [Google Scholar]
- 13.Vakil N. Helicobacter pylori treatment: A practical approach. Am J Gastroenterol. 2006;101:497–9. doi: 10.1111/j.1572-0241.2006.00454.x. [DOI] [PubMed] [Google Scholar]
- 14.McNicholl AG, Marin AC, Molina-Infante J, Castro M, Barrio J, Ducons J, et al. Participant Centres. Randomised clinical trial comparing sequential and concomitant therapies for Helicobacter pylori eradication in routine clinical practice. Gut. 2014;63:244–9. doi: 10.1136/gutjnl-2013-304820. [DOI] [PubMed] [Google Scholar]
- 15.Liou JM, Chen CC, Chen MJ, Chen CC, Chang CY, Fang YJ, et al. Taiwan Helicobacter Consortium. Sequential versus triple therapy for the first-line treatment of Helicobacter pylori: A multicentre, open-label, randomised trial. Lancet. 2013;381:205–13. doi: 10.1016/S0140-6736(12)61579-7. [DOI] [PubMed] [Google Scholar]
- 16.Agheyisi R. Norwalk, CT, USA: Technical Report, Business Communication Company; 2005. Ga-121 Probiotics: Ingredients, Supplements, Foods; pp. 2–5. [Google Scholar]
- 17.Mainville I, Arcand Y, Farnworth ER. A dynamic model that simulates the human upper gastrointestinal tract for the study of probiotics. Int J Food Microbiol. 2005;99:287–96. doi: 10.1016/j.ijfoodmicro.2004.08.020. [DOI] [PubMed] [Google Scholar]
- 18.Desmond C, Stanton C, Fitzgerald GF, Collins K, Ross RP. Environmental adaptation of probiotic lactobacilli towards improvement of performance during spray drying. Int Dairy J. 2001;11:801–8. [Google Scholar]
- 19.Hansen TL, Allan-Wojtas PM, Jin YL, Paulson AT. Survival of Ca-alginate microencapsulated Bifidobacterium spp.in milk and simulated gastrointestinal conditions. Food Microbiol. 2002;19:35–45. [Google Scholar]
- 20.Capela P, Hay TK, Shah NP. Effect of cryoprotectants, prebiotics and microencapsulation on survival of probiotic organisms in yoghurt and freeze-dried yoghurt. Food Res Inter. 2006;39:203–11. [Google Scholar]
- 21.Gbassi GK, Vandamme T, Ennahar S, Marchioni E. Microencapsulation of Lactobacillus plantarum spp in an alginate matrix coated with whey proteins. Int J Food Microbiol. 2009;129:103–5. doi: 10.1016/j.ijfoodmicro.2008.11.012. [DOI] [PubMed] [Google Scholar]
- 22.Heidebach T, Först P, Kulozik U. Influence of casein-based microencapsulation on freeze-drying and storage of probiotic cells. J Food Eng. 2010;98:309–16. [Google Scholar]
- 23.Ray B, Daeschel MA. Bacteriocins of starter culture bacteria. In: Dillon VM, Board RG, editors. Natural Antimicrobial Systems and Food Preservation. Oxon, UK: CAB International; 1994. pp. 133–65. [Google Scholar]
- 24.Kaur B, Balgir PP. Biopreservative potential of a broad-range pediocin CP2 obtained from Pediococcus acidilactici MTCC 5101. Asian J Microbiol Biotechnol Environ Sci. 2008;10:439–44. [Google Scholar]
- 25.Prema P, Smila D, Palavesam A, Immanuel G. Production and characterization of an antifungal compound (3-phenyllactic acid) produced by Lactobacillus plantarum strain. Food Bioprocess Technol. 2008;3:379–86. [Google Scholar]
- 26.El-Adawi H, Khalil AM, El-Sheekh M, El-Deeb NM, Hussein MZ. Cytotoxicity assay and antioxidant activities of the lactic acid bacterial strains. Afr J Microbiol Res. 2012;6:1700–12. [Google Scholar]
- 27.El-Adawi H, El-Sheekh M, Khalil M, El-Deeb N, Hussein M. Lactic acid bacterial extracts as anti-Helicobacter pylori: A molecular approach. Ir J Med Sci. 2013;182:439–52. doi: 10.1007/s11845-013-0909-y. [DOI] [PubMed] [Google Scholar]
- 28.Krasaekoopt W, Bhandari B, Deeth HC. Survival of probiotics encapsulated in chitosan-coated alginate beads in yoghurt from UHT- and conventionally treated milk during storage. LWT- Food Sci Technol. 2006;39:177–83. [Google Scholar]
- 29.Rosenberg M, Kopelman IJ, Talmon Y. A Scanning electron microscopy study of microencapsulation. J Food Sci. 1985;50:139–44. [Google Scholar]
- 30.Kasra-Kermanshahi R, Fooladi J, Peymanfar S. Isolation and microencapsulation of Lactobacillus spp.from corn silage for probiotic application. Iran J Microbiol. 2010;2:98–102. [PMC free article] [PubMed] [Google Scholar]
- 31.Chen H, Ouyang W, Jones M, Haque T, Lawuyi B, Prakash S. In-vitro analysis of APA microcapsules for oral delivery of live bacterial cells. J Microencapsul. 2005;22:539–47. doi: 10.1080/02652040500162162. [DOI] [PubMed] [Google Scholar]
- 32.Berrada N, Lemeland JF, Laroche G, Thouvenot P, Piaia M. Bifidobacterium from fermented milks: Survival during gastric transit. J Dairy Sci. 1991;74:409–13. doi: 10.3168/jds.S0022-0302(91)78183-6. [DOI] [PubMed] [Google Scholar]
- 33.Shapiro SS, Wilk MB. An analysis of variance test for normality (complete samples) Biometrika. 1965;52:591–611. [Google Scholar]
- 34.Cramer D, Howitt D. London: SAGE Publication, 2004; 2004. The SAGE Dictionary of Statistics. doi: http://dx.doi.org/10.4135/9780857020123 . [Google Scholar]
- 35.Kailasapathy K. Microencapsulation of probiotic bacteria: Technology and potential applications. Curr Issues Intest Microbiol. 2002;3:39–48. [PubMed] [Google Scholar]
- 36.Chang TM. Bioencapsulation in biotechnology. Biomater Artif Cells Immobil Biotechnol. 1993;21:291–7. doi: 10.3109/10731199309117366. [DOI] [PubMed] [Google Scholar]
- 37.Chang TM. Semipermeable microcapsules. Science. 1964;146:524–5. doi: 10.1126/science.146.3643.524. [DOI] [PubMed] [Google Scholar]
- 38.Chang TM. Therapeutic applications of polymeric artificial cells. Nat Rev Drug Discov. 2005;4:221–35. doi: 10.1038/nrd1659. [DOI] [PubMed] [Google Scholar]
- 39.Chang TM, Prakash S. Therapeutic uses of microencapsulated genetically engineered cells. Mol Med Today. 1998;4:221–7. doi: 10.1016/s1357-4310(98)01246-5. [DOI] [PubMed] [Google Scholar]
- 40.Kovacs-Nolan J, Mine Y. Microencapsulation for the gastric passage and controlled intestinal release of immunoglobulin Y. J Immunol Methods. 2005;296:199–209. doi: 10.1016/j.jim.2004.11.017. [DOI] [PubMed] [Google Scholar]
- 41.Narayani R, Rao KP. Polymer-coated gelatin capsules as oral delivery devices and their gastrointestinal tract behavior in humans. J Biomat Sci Polym Ed. 1995;7:39–48. doi: 10.1163/156856295x00814. [DOI] [PubMed] [Google Scholar]
- 42.Sato Y, Kawashima Y, Takeuchi H, Yamamoto H, Fujibayashi Y. Pharmacoscintigraphic evaluation of riboflavin-containing microballoons for a floating controlled drug delivery system in healthy humans. J Control Release. 2004;98:75–85. doi: 10.1016/j.jconrel.2004.04.021. [DOI] [PubMed] [Google Scholar]
- 43.Lamprecht A, Yamamoto H, Takeuchi H, Kawashima Y. Design of pH-sensitive microspheres for the colonic delivery of the immunosuppressive drug tacrolimus. Eur J Pharm Biopharm. 2004;58:37–43. doi: 10.1016/j.ejpb.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 44.Delie F. Evaluation of nano- and microparticle uptake by the gastrointestinal tract. Adv Drug Deliv Rev. 1998;34:221–33. doi: 10.1016/s0169-409x(98)00041-6. [DOI] [PubMed] [Google Scholar]
- 45.Bhatia SR, Khattak SF, Roberts SC. Polyelectrolytes for cell encapsulation. Curr Opin Colloid Interface Sci. 2005;10:45–51. [Google Scholar]
- 46.Conway PL, Kjelleberg S. Protein-mediated adhesion of Lactobacillus fermentum strain 737 to mouse stomach squamous epithelium. J Gen Microbiol. 1989;135:1175–86. doi: 10.1099/00221287-135-5-1175. [DOI] [PubMed] [Google Scholar]
- 47.Lesbros D, Corthesy-Theulaz I, Dorta G, Stolte M, Isler P, Rochat F. Favorable effect of long-term intake of fermented milk containing Lactobacillus johnsonii on H. pylori associated gastritis. Aliment Pharmacol Ther. 2003;18:805–13. doi: 10.1046/j.1365-2036.2003.01675.x. [DOI] [PubMed] [Google Scholar]
- 48.Michetti P, Dorta G, Wiesel PH, Brassart D, Verdu E, Herranz M, et al. Effect of whey-based culture supernatant of Lactobacillus acidophilus (johnsonii) La1 on Helicobacter pylori infection in humans. Digestion. 1999;60:203–9. doi: 10.1159/000007660. [DOI] [PubMed] [Google Scholar]
- 49.Cats A, Kuipers EJ, Bosschaert MA, Pot RG, Vandenbroucke-Grauls CM, Kusters JG. Effect of frequent consumption of a Lactobacillus casei-containing milk drink in Helicobacter pylori-colonized subjects. Aliment Pharmacol Ther. 2003;17:429–35. doi: 10.1046/j.1365-2036.2003.01452.x. [DOI] [PubMed] [Google Scholar]
- 50.Linsalata M, Russo F, Berloco P, Caruso ML, Matteo GD, Cifone MG, et al. The influence of Lactobacillus brevis on ornithine decarboxylase activity and polyamine profiles in Helicobacter pylori-infected gastric mucosa. Helicobacter. 2004;9:165–72. doi: 10.1111/j.1083-4389.2004.00214.x. [DOI] [PubMed] [Google Scholar]
- 51.Sakamoto I, Igarashi M, Kimura K, Takagi A, Miwa T, Koga Y. Suppressive effect of Lactobacillus gasseri OLL 2716 (LG21) on Helicobacter pylori infection in humans. J Antimicrob Chemother. 2001;47:709–10. doi: 10.1093/jac/47.5.709. [DOI] [PubMed] [Google Scholar]
- 52.Hirayama K, Rafter J. The role of lactic acid bacteria in colon cancer prevention: Mechanistic considerations. Antonie Van Leeuwenhoek. 1999;76:391–4. doi: 10.1007/978-94-017-2027-4_25. [DOI] [PubMed] [Google Scholar]
- 53.Zimmermann H, Zimmermann D, Reuss R, Feilen PJ, Manz B, Katsen A, et al. Towards a medically approved technology for alginate-based microcapsules allowing long-term immunoisolated transplantation. J Mater Sci Mater Med. 2005;16:491–501. doi: 10.1007/s10856-005-0523-2. [DOI] [PubMed] [Google Scholar]
- 54.Smoot DT. How does Helicobacter pylori cause mucosal damage? Direct mechanisms. Gastroenterology. 1997;113(Suppl):S31–4. doi: 10.1016/s0016-5085(97)80008-x. discussion S50. [DOI] [PubMed] [Google Scholar]
- 55.Coconnier MH, Lievin V, Hemery E, Servin AL. Antagonistic activity against Helicobacter infection in vitro and in vivo by the human Lactobacillus acidophilus strain LB. Appl Environ Microbiol. 1998;64:4573–80. doi: 10.1128/aem.64.11.4573-4580.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nam H, Ha M, Bae O, Lee Y. Effect of Weissella confusa strain PL9001 on the adherence and growth of Helicobacter pylori. Appl Environ Microbiol. 2002;68:4642–5. doi: 10.1128/AEM.68.9.4642-4645.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Johnson-Henry KC, Mitchell DJ, Avitzur Y, Galindo-Mata E, Jones NL, Sherman PM. Probiotics reduce bacterial colonization and gastric inflammation in H. pylori-infected mice. Dig Dis Sci. 2004;49:1095–102. doi: 10.1023/b:ddas.0000037794.02040.c2. [DOI] [PubMed] [Google Scholar]
- 58.Aiba Y, Suzuki N, Kabir AM, Takagi A, Koga Y. Lactic acid-mediated suppression of Helicobacter pylori by the oral administration of Lactobacillus salivarius as a probiotic in a gnotobiotic murine model. Am J Gastroenterol. 1998;93:2097–101. doi: 10.1111/j.1572-0241.1998.00600.x. [DOI] [PubMed] [Google Scholar]


