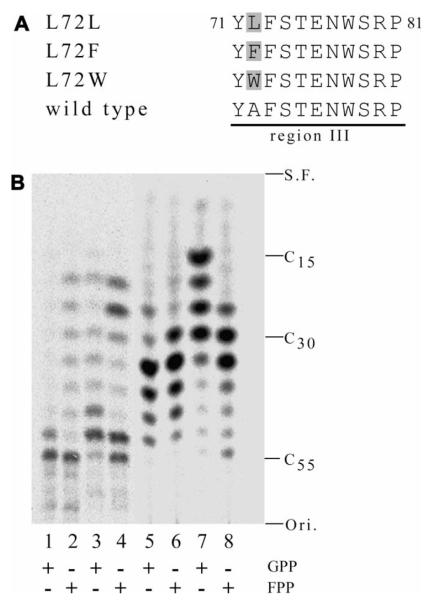
Fig. 2.
Introduction of substitution mutations into region III of UPS from M. luteus B-P 26. (A) Partial amino acid sequences around region III of wild type and mutant enzymes are aligned. The substituted amino acid residues are shaded. (B) TLC autoradiochromatogram of the reaction products of wild type and mutant enzymes. Lanes 1 and 2, wild type; lanes 3 and 4, A72L; lanes 5 and 6, A72F; and lanes 7 and 8, A72W. The products were analyzed as described in Materials and methods. Each reaction mixture contained 10 μM GPP (lanes 1, 3, 5, and 7) or E,E-FPP (lanes 2, 4, 6, and 8) as an allylic substrate. Under all assay conditions, less than 30% of each substrate reacted. Ori., origin; S.F., solvent front.