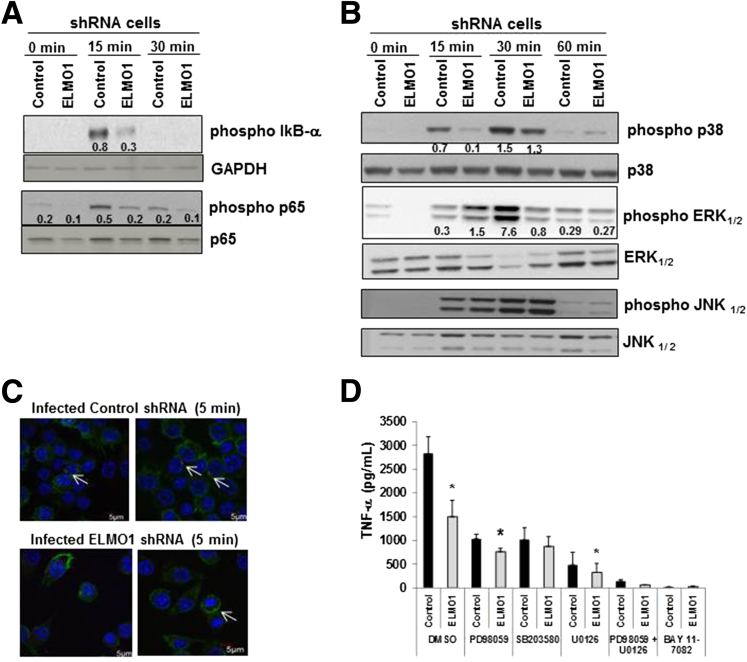
Figure 4.
Engulfment and cell motility protein 1 (ELMO1)-mediated tumor necrosis factor-α (TNF-α) responses are induced via the NF-κB and MAP kinase pathway. (A) Role of ELMO1 in nuclear factor κB (NF-κB) activation evaluated in control and ELMO1 small-hairpin RNA (shRNA) cells by immunoblotting phospho-IκB and phospho p65 after Salmonella infection for the indicated time. The same blot was stripped and reprobed with antibodies to detect the glyceraldehyde-3-phosphate dehydrogenase (phosphorylating) (GAPDH) and total p65. (B) Control and ELMO1 shRNA cells incubated with Salmonella (SL1344) for indicated time points. Cells were harvested and lysed, and Western blots were performed with phospho-specific antibodies to detect p38 mitogen-activated protein kinase (MAPK), extracellular signal-regulated kinases 1/2 (ERK1/2), and c-Jun N-terminal kinase 1/2 (JNK1/2). A transient increase in p38 MAPK phosphorylation (15 minutes and 30 minutes) and ERK1/2 (30 minutes) was observed in control cells compared with ELMO1 shRNA cells. The figures in A and B represent three independent experiments. (C) Bacterial internalization confirmed using confocal microscopy after infection of control and ELMO1 shRNA cells with SL1344-RFP (red) after 5 minutes of infection. The actin of the macrophages was stained with phalloidin (green), and the nuclei was stained with Hoechst (blue). (D) To investigate the effect of p38 MAPK and ERK1/2 kinases, control and ELMO1 shRNA cells infected with Salmonella (SL1344) for 3 hours in the presence and absence of p38 MAPK inhibitor SB203580 (10 μM), ERK1/2 inhibitor PD98059 (50 μM), and U0126 (10 μM), and NF-κB inhibitor BAY 11–7082 (10 μM). Subsequently, supernatants were assayed for TNF-α by enzyme-linked immunosorbent assay. Data represent the mean ± standard deviation of three separate experiments. *P ≤ .05, compared with the respective control shRNA, two-tailed Student t test.