Fig. 2. Mitochondrial Parkin mobilization directed by pseudo-PINK1 phosphorylated Mfn2.
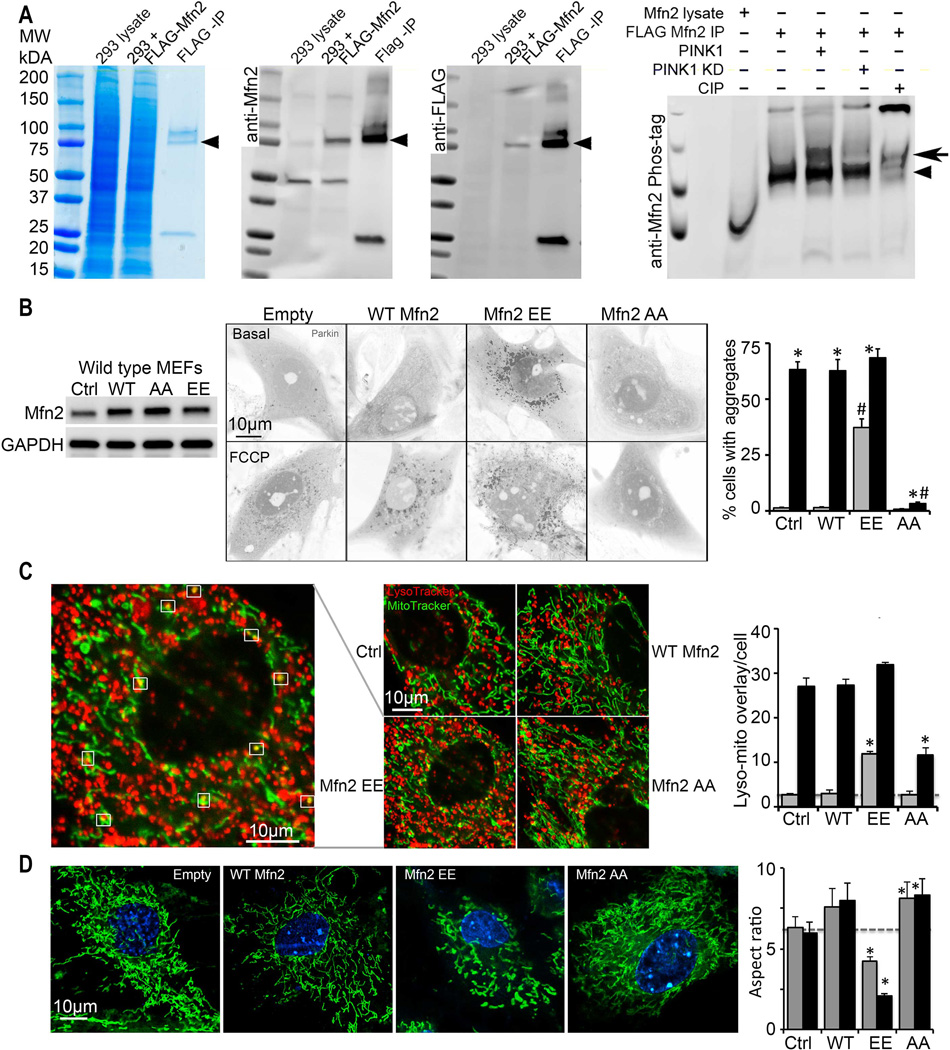
(A) Phosphorylation of Mfn2 by recombinant PINK1 in a cell-free system. First three panels show enrichment of FLAG-Mfn2 by anti-FLAG immunoprecipitation (IP); left is Coomassie blue stained gel, middle is anti-Mfn2 immunoblot, right is anti-FLAG immunoblot. Fourth panel shows anti-Mfn2 Phos-Tag immunoblot of in vitro PINK1 phosphorylation reactants; KD is kinase dead PINK1, CIP is calf intestinal phosphatase. Arrowheads show FLAG-Mfn2; bold arrow indicates phospho-Mfn2. (B) Spontaneous mcParkin translocation in MEFs provoked by adeno-Mfn2 EE, and FCCP-mediated Parkin translocation suppressed by adeno-Mfn2 AA. To the left is immunoblot of Mfn2. (C) Lysosomal-mitochondrial interactions (white squares) provoked by adeno-Mfn2 EE and suppressed by adeno-Mfn2 AA. (D) Mitochondrial elongation (aspect ratio) inhibited by adeno-Mfn2 EE and stimulated by adeno-Mfn2 AA. WT is wild type adeno-Mfn2. In B and C grey bars are basal; black bars are after FCCP or antimycin A. In D grey bars are 24h and black bars are 48h after adeno-Mfn virus infection. * is p<0.05 vs adeno β-gal control (Ctrl); # is p<0.05 vs same condition WT adeno-Mfn2.
