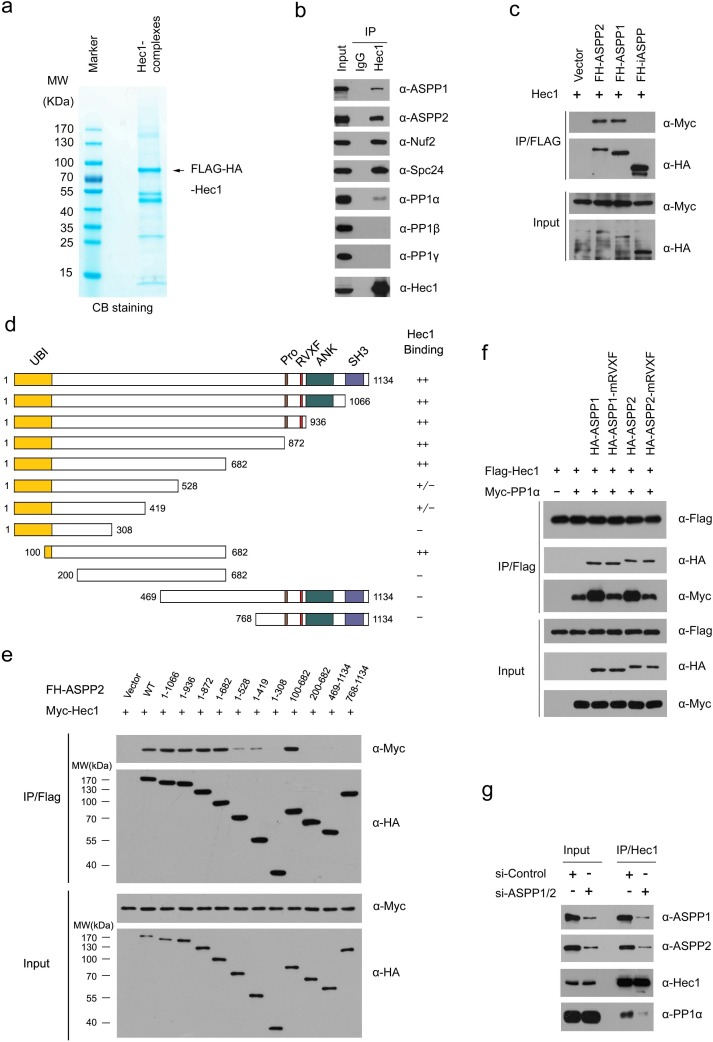
Figure 5. ASPP1/2 facilitate the interaction between Hec1 and PP1α.
a. Tandem affinity purification of the Hec1-containing protein complex was conducted using HeLa cells stably expressing FLAG-HA (FH)-Hec1. Associated proteins were separated by SDS-PAGE and visualized by CB staining. The proteins and the number of peptides identified by mass spectrometry analysis are shown in the Supplementary Table S3. b. Endogenous Hec1 interaction with ASPP1/2 and PP1α. Immunoprecipitation with anti-Hec1 antibody was performed using cell lysates prepared from HeLa cells. The presence of proteins in the immunoprecipitates was detected by WB analyses using the indicated antibodies. c. iASPP cannot interact with Hec1. 293T cells were co-transfected with Myc-Hec1 and FH-ASPP (ASPP1, ASPP2 or iASPP) constructs. After 24 hr, cell lysates were prepared for immunoprecipitation with the anti-Flag antibody and detected by WB analyses using the indicated antibodies. d. Schematic representation of ASPP2 deletion mutants. Binding capacity of ASPP2 WT or mutants to Hec1 is indicated with the symbols. e. Identification of Hec1-binding domain in ASPP2. 293T cells were co-transfected with Myc-Hec1 and FH-ASPP2-WT or deletion mutants. After 24 hr, cell lysates were prepared for immunoprecipitation with anti-FLAG antibody and detected by WB analyses. f. ASPP1/2 facilitate the interaction between Hec1 and PP1α in a PP1-binding dependent manner. 293T cells were co-transfected with indicated constructs. After 24 hr, cell lysates were prepared for immunoprecipitation with the anti-Flag antibody and detected by WB analyses using indicated antibodies. g. ASPP1/2 co-depletion reduces the endogenous interaction between Hec1 and PP1α. HeLa cells were transfected with the control or ASPP1/2 siRNAs. After 48 hr, cell lysates were prepared for immunoprecipitation with anti-Hec1 antibody and detected by WB analyses using the indicated antibodies.