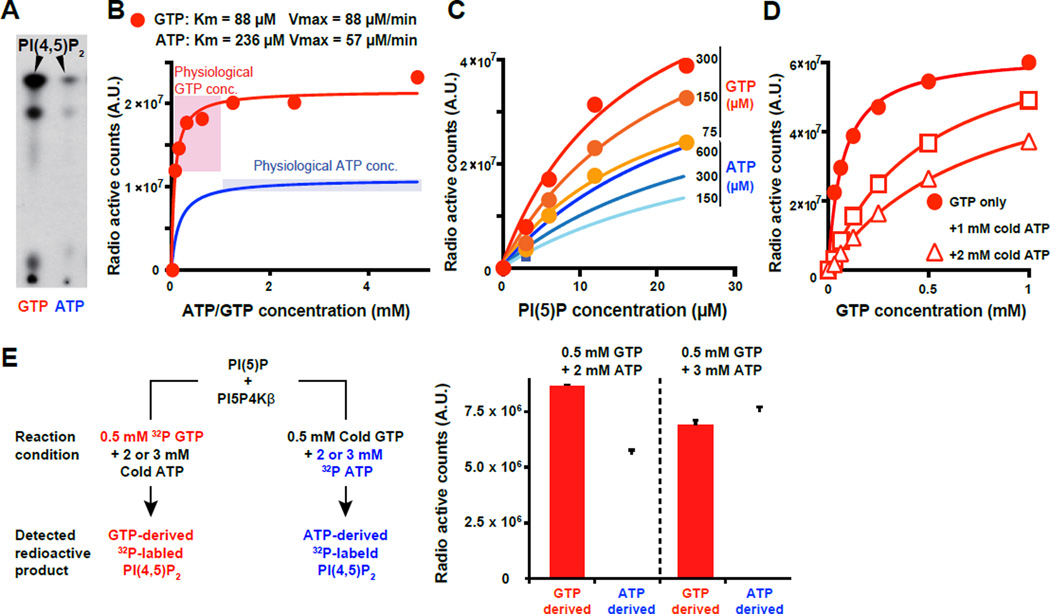
Figure 2. PI5P4Kβ has ability to sense GTP concentration.
(A) PI5P4Kβ utilizes GTP as a preferred phosphodonor to produce PI(4,5)P2 from PI(5)P. The bacterially produced recombinant PI5P4Kβ was incubated with PI(5)P in the presence of 0.2 µM γ-32P radiolabeled GTP or ATP. The phospholipids were extracted and analyzed by thin layer chromatography and autoradiography.
(B) PI5P4Kβ activity changes within the physiological variations of GTP concentration. PI5P4Kβ kinase activity was assessed with the indicated levels of γ-32P radiolabeled GTP or ATP and 20 µM of PI(5)P.
(C) PI5P4Kβ retains its preference to GTP at any PI(5)P concentration.
(D) PI5P4Kβ retains GTP responsiveness in the presence of physiological amount of ATP. PI5P4Kβ kinase activity with the indicated amount of γ-32P radiolabeled GTP was assessed in the presence of 1 mM and 2 mM of cold (non-radiolabeled) ATP.
(E) PI5P4Kβ efficiently utilizes GTP in the presence of physiological amount of ATP. GTP- and ATP-dependent kinase activities of PI5P4Kβ were assessed with mixtures of cold and γ-32P radiolabeled ATP and GTP as shown in the left panel. 0.2 µg PI5P4Kβ and 1.0 µg PI(5)P was used for each reaction and only radiolabeled PI(4,5)P2 was quantified (right panel). Data are displayed as means ± SD, n=3.
See also Figure S2.