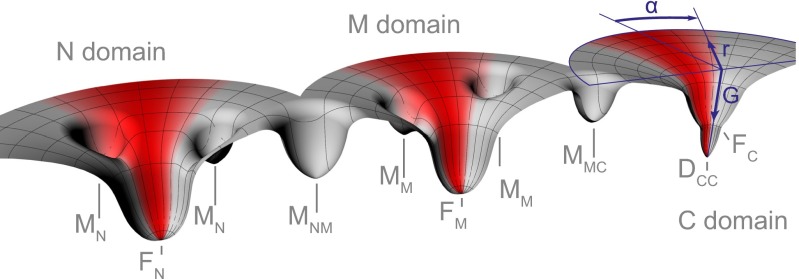
Fig. 5.
Simplified energy landscapes for folding and assembly of Hsp90. The folding properties of Hsp90 can be described by three individual energy landscapes, because the individual domains don’t directly stabilize each other. Experiments using the individual domains showed that the N and the M domain have a fast productive folding pathway to the native states (FN, FM) but that the overall folding rate is greatly slowed down by off-pathway intermediates (MN, MM). The C domain exhibits two-state behavior without kinetic traps. Refolding of the full-length monomer showed heterogeneous and stable cross-domain misfolds (MNM, MMC). A cylindrical coordinate system shown for the C domain (blue) applies for all domains. G refers to free energy. The inverse of the radial coordinate r describes the overall number of residues with native conformation. The inverse of the absolute value of the angular coordinate α can be interpreted as the average distance between residues in misfolded conformations. This distance is strongly dependent on force and restricts the conformational search (red shaded areas), speeding up folding by avoiding or depopulating misfolded species. This effect is particularly strong for the cross-domain misfolds in the full-length monomer. After successful formation of the C domain, Hsp90 can dimerize into a functional chaperone (DCC).