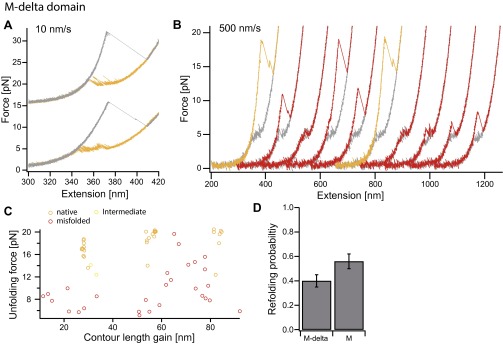
Fig. S3.
Folding behavior of the C-terminally trancated mutant of the M domain. Force-extension cycles of the M-delta (10 amino acids less at the C-terminal end) construct at a velocity of 10 nm/s (A) and 500 nm/s (B). The on-pathway intermediate observed in the M domain construct seems to be less stable while refolding in slow traces (see Fig. 2B and Fig. S2C). Faster pulled traces that allow probing of the misfolded states rarely populate the intermediate state alone (compare with yellow curves in Fig. S2D). This is also observed in a scatter plot of 46 unfolding traces pulled at 500 nm/s (C); compare to Fig. 2E. (D) Refolding probabilities of force-extension cycles at 500 nm/s for the M-delta and the M domain. The refolding probability is 40 ± 5% for the M-delta domain and 56 ± 6% for the M domain (calculated from probabilities of 10/11 molecules, a total of 304/414 unfolding traces for the M-delta/M domain. Errors are SD). Whether the decreased refolding probability is due to terminal residues that are missing and involved in domain formation or due to a decrease in refolding rate because the spatially close ubiquitin hampers folding is not apparent. However, it is obvious that the folding properties of the intermediate have changed, suggesting that the intermediate results from the small alpha/beta/alpha subdomain at the C terminus of the M domain.