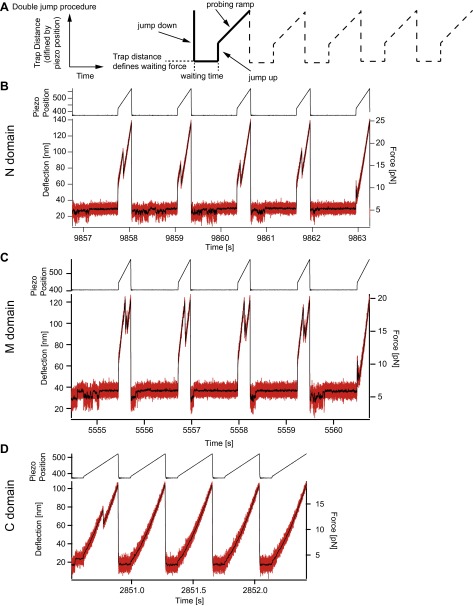
Fig. S4.
Double jump assay and raw data examples of the individual domains. (A) For well-defined refolding parameters and an easy readout of the protein response, a special force exertion pattern is applied. The completely unfolded peptide is relaxed as rapidly as possible (jump down) to a certain trap distance. From the DNA and protein parameters, the waiting force is calculated. The waiting force is the force that acts on the protein if the protein chain is completely unfolded. After a certain waiting time, the trap distance is increased (jump up) to forces that quench the refolding process but don’t unfold the native structure. In a subsequent probing ramp, the state of the protein is evaluated. (B−D) Example raw data of the double-jump experiments of the N domain (B), the M domain (C), and the C domain (D). From the probing ramps, force-extension traces are calculated and the state of the protein is determined. At high-enough waiting forces during the waiting time, we can directly observe the folding of the molecules (see also Fig. S5).