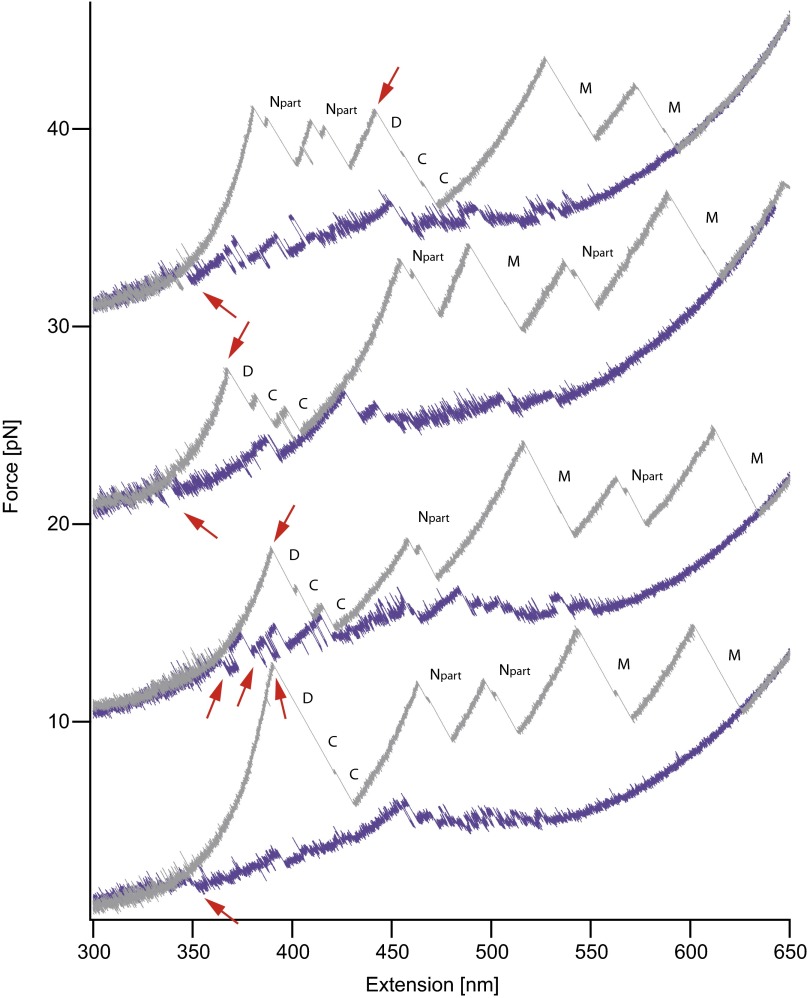
Fig. S8.
Additional dimer force-extension traces with identical conditions to that shown in Fig. 4. Each stretch and relax cycle is of a different molecule. We always observe dimer dissociation in unfolding traces, as well as association in refolding traces (red arrows). In the first trace, both of the N domains unfold before the dimer is separated. The shortened N domain (Npart) is due to pulling at amino acid positions 61. In this pulling geometry, an intermediate in the N domain is populated. The same contour length gain and intermediate were also observed in a monomeric construct pulled at residues 61 and 560. Traces are shown in the supplement of ref. 13.