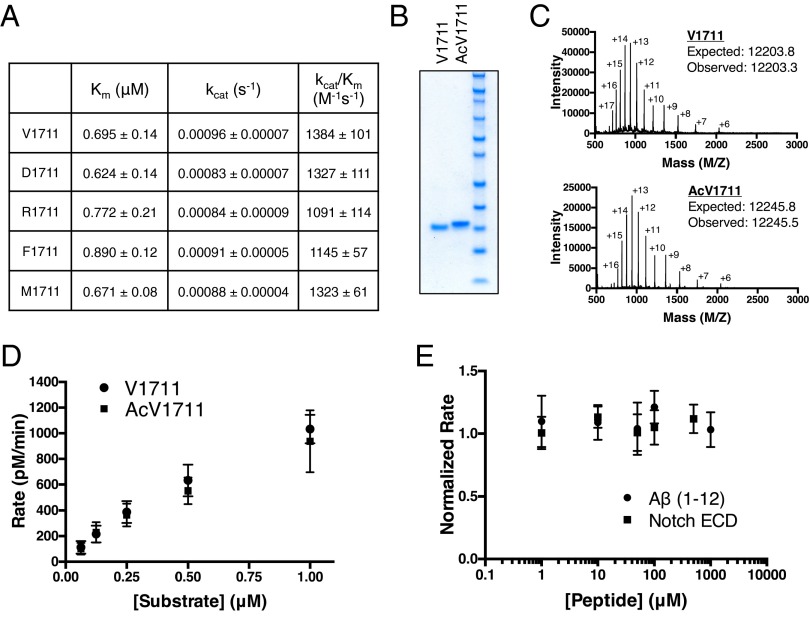
Fig. 1.
γ-secretase activity toward N-terminally manipulated notch. (A) Summary of kinetic data from γ-secretase cleavage of notch substrates containing varying N-terminal amino acids. Cleavage was monitored by Western blot using a cleavage-specific antibody to NICD (mean ± SD, n = 3). (B) Coomassie-stained gel of semisynthetic native V1711 and N-terminally acetylated AcV1711 generated by native chemical ligation. (C) Electrospray ionization time-of-flight mass spectrometry of V1711 and AcV1711. Intact masses were obtained after deconvolution of the multiply charged states of each protein. (D) γ-secretase activity toward semisynthetic V1711 and AcV1711 (mean ± SD, n = 2). (E) In trans peptide inhibition of recombinant V1711 with notch ectodomain and Aβ (1–12) peptides. Data points are normalized to untreated V1711 cleavage (mean ± SD, n = 2).