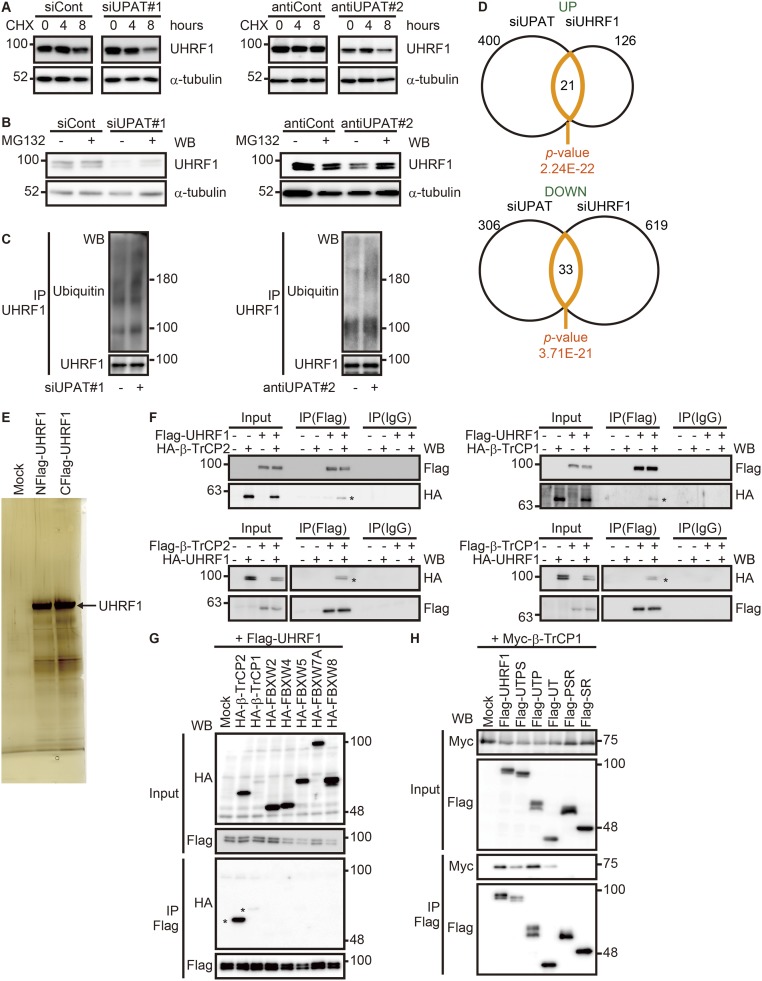
Fig. S4.
UPAT stabilizes UHRF1 protein by interfering with its proteasome-mediated ubiquitination and degradation. (A) HCT116 cells transfected with siRNA targeting UPAT (Left, siUPAT#1) or an antisense oligonucleotide targeting UPAT (Right, antiUPAT#2) were treated with CHX for the indicated times and then subjected to immunoblotting analysis with anti-UHRF1 or anti–α-tubulin antibody. α-tubulin was used as a loading control. (B) HCT116 cells transfected with siRNA targeting UPAT (siUPAT#1) or an antisense oligonucleotide targeting UPAT (Right, antiUPAT#2) were cultured in the presence or absence of MG132 and then subjected to immunoblotting analysis with anti-UHRF1 or anti–α-tubulin antibody. α-tubulin was used as a loading control. (C) UPAT inhibits polyuiquitination of UHRF1. Lysates from HCT116 cells that had been transfected with siRNA targeting UPAT (siUPAT#1) or an antisense oligonucleotide targeting UPAT (Right, antiUPAT#2) and treated with MG132 were subjected to immunoprecipitation with anti-UHRF1 antibody followed by immunoblotting analysis with anti-ubiquitin or anti-UHRF1 antibody. (D) Venn diagram showing the overlap between genes regulated by knockdown of UPAT and those regulated by knockdown of UHRF1. The P value for the significance of the overlap is 2.24E-22 for up-regulated genes (UP) and 3.71E-21 for down-regulated genes (DOWN) as determined by hypergeometric distribution. (E) Lysates from HCT116 cells transfected with UHRF1 tagged at its NH2- or COOH terminus were subjected to immunoprecipitation with anti-Flag antibody and resolved by SDS/PAGE followed by silver staining. The arrowhead indicates UHRF1. The immunoprecipitates were subjected to liquid chromatography–mass spectrometry. (F, Upper) Lysates from HCT116 cells that had been transfected with HA-tagged β-TrCP2 (Left) or β-TrCP1 (Right) and/or Flag-tagged UHRF1 and treated with MG132 were immunoprecipitated (IP) with anti-Flag antibody followed by immunoblotting analysis with anti-HA or anti-Flag antibody. (Lower) 293FT cells transfected with Flag-tagged β-TrCP2 (Left) or β-TrCP1 (Right) and/or HA-tagged UHRF1 were treated with MG132 and then subjected to immunoprecipitation (IP) with anti-Flag antibody followed by immunoblotting analysis with anti-HA or anti-Flag antibody. (G) Lysates from 293FT cells transfected with Flag-tagged UHRF1 along with empty vector (Mock) or the indicated HA-tagged F-box protein expression constructs were immunoprecipitated (IP) with anti-Flag antibody followed by immunoblotting analysis with anti-HA or anti-Flag antibody. (H) Lysates from 293FT cells transfected with Myc-tagged β-TrCP1 along with empty vector (Mock) or mutants of UHRF1 were immunoprecipitated (IP) with anti-Flag antibody followed by immunoblotting analysis with anti-Myc or anti-Flag antibody.